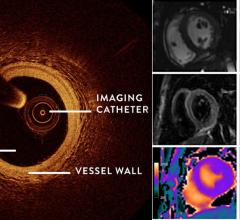
May 13, 2021 — The Centers for Disease Control and Prevention (CDC) just released a new statement relaxing the ...
Magnetic Resonance Imaging (MRI)
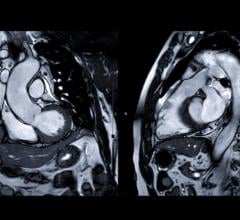
Cardiac MRI creates images from the resonance of hydrogen atoms when they are polarized to face in one direction and then hit with an electromagnetic pulse to knock them off axis. The wobbling of the atoms is what is recorded by computers and used to reconstruct the images. Cardiac MR allows very detailed visualization of the myocardial tissue above the resolution found with cardiac CT. Using different protocol sequences, various contrast type images can be created with MRI to enhance various tissues or to provide physiological data on the function of the heart. This section includes MR analysis software, MRI scanners, gadolinium contrast agents, and related magnetic resonance accessories.
May 12, 2021 — According to the British Heart Foundation, heart and circulatory diseases cause more than a quarter (27 ...
May 11, 2021 — The American Institute of Ultrasound in Medicine (AIUM) and the American Society of Echocardiography (ASE ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
March 17, 2021 — Around 50% of patients who have been hospitalized with severe COVID-19 (SARS-CoV-2) and have damage to ...
March 3, 2021 — Impulse Dynamics recently announced that DEKRA Certification B.V., a global regulatory organization ...
January 11, 2021 — In a letter published in the December issue of the American Heart Association (AHA) medical journal C ...
November 17, 2020 — Diagnostic imaging techniques were able to find the underlying cause of heart attack in many women ...
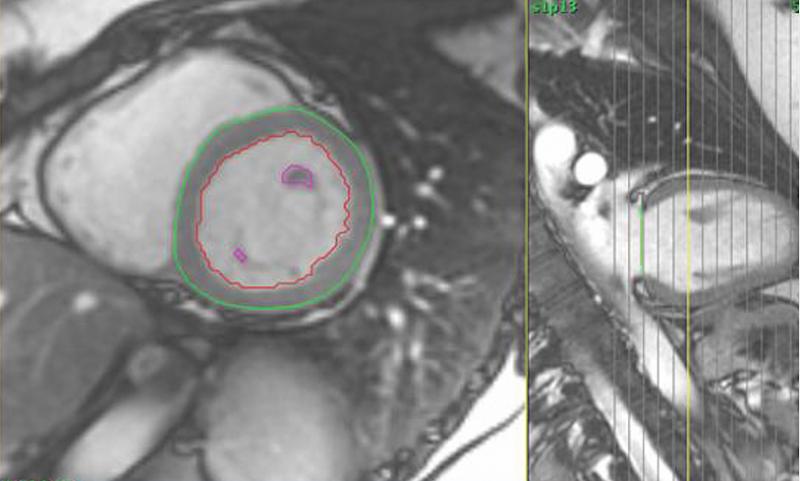
October 29, 2020 — Contrast agents used to improve views of the heart on magnetic resonance imaging (MRI) carry a very ...
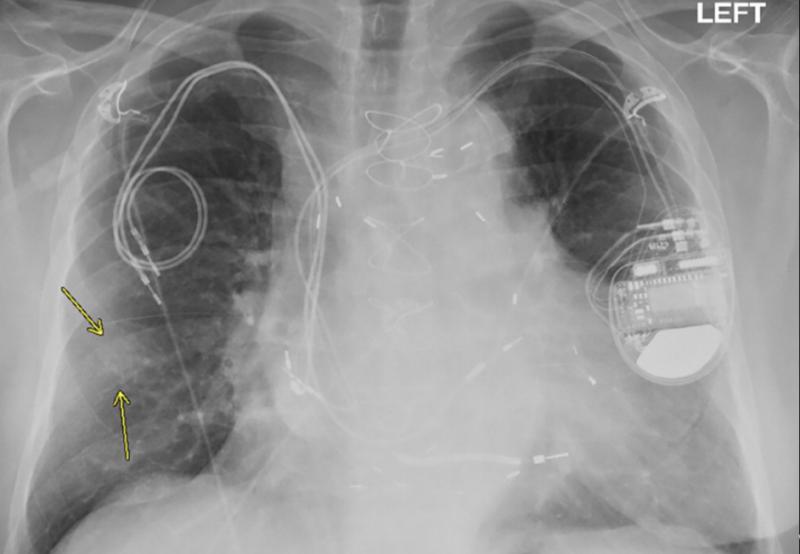
October 27, 2020 – Magnetic resonance imaging (MRI) examinations can be safely performed in patients with non-MR ...
Advanced visualization company Medis recently purchased Advanced Medical Imaging Development S.r.l. (AMID), which ...
August 11, 2020 — Myocardial Solutions, Inc. and United Imaging, Inc. have formed a strategic partnership to open new ca ...
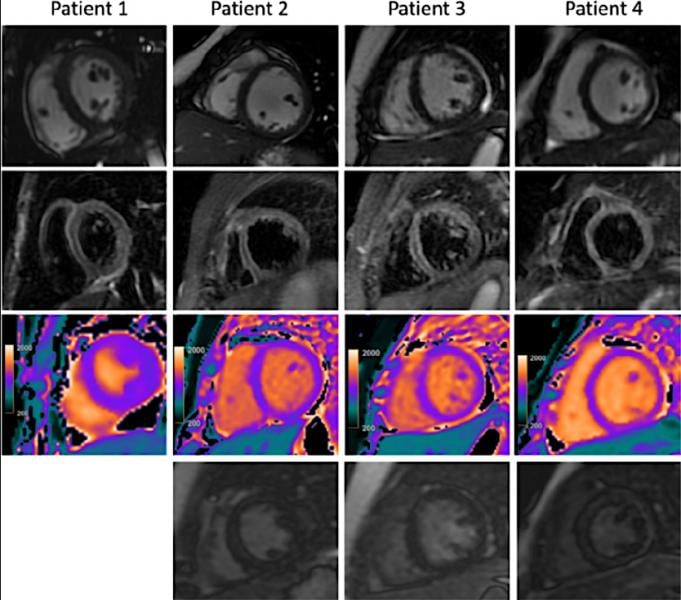
July 8, 2020 — Cardiac magnetic resonance imaging (MRI) has been shown to be helpful in evaluating multisystem ...
June 29, 2020 — A type of smart magnetic resonance imaging (MRI) scan used in people with heart disease could help ...
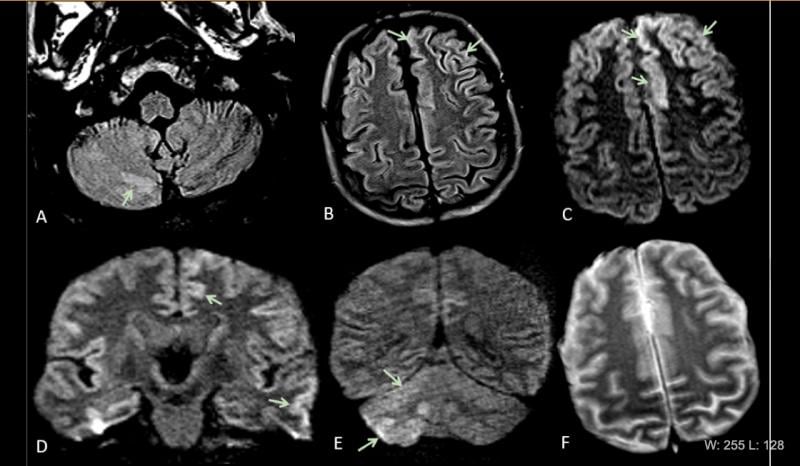
June 2, 2020 — Four recent radiology studies, from New York, Italy, Iran and China, show how COVID-19 (SARS-CoV-2) impac ...
May 27, 2020 — The Radiological Society of North America (RSNA) announced today that its 106th Scientific Assembly and ...


 May 13, 2021
May 13, 2021