Ajay J. Kirtane, M.D., associate professor of medicine at Columbia University Irving Medical Center and director of the ...
Stents Drug Eluting
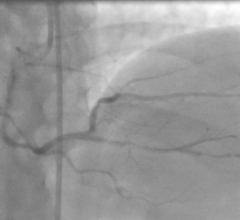
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
Roxana Mehran, M.D., FACC, FACP, FCCP, FESC, FAHA, FSCAI, professor of medicine and director of interventional ...
American Heart Association President Robert Harrington, M.D., interventional cardiologist and the Arthur L. Bloomfield ...
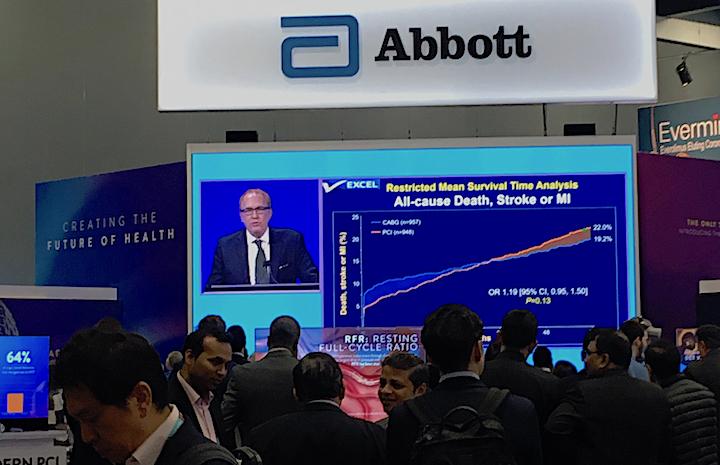
October 3, 2019 – Five-year data from the EXCEL Trial showed patients with left main coronary disease treated with ...
September 30, 2019 – The first randomized trial to compare a durable polymer drug-eluting stent to a polymer-free drug ...
September 30, 2019 — A biodegradable polymer everolimus-eluting stent (BP-EES) followed by four months of dual ...
September 25, 2019 — Ten-year survival rates are similar for bypass surgery and coronary stenting with drug-eluting ...
September 5, 2019 — Biotronik's ultrathin Orsiro stent demonstrated superiority over Xience with respect to target ...

August 28, 2019 — The U.S. Food and Drug Administration (FDA) released an updated MedWatch Alert this month on the ...
August 21, 2019 — Increasing incidence of cardiovascular diseases worldwide is creating growth opportunities for the ...
April 3, 2019 — Medtronic's Resolute Integrity Zotarolimus-eluting Coronary Stent System received an additional U.S ...

February 22, 2019 — The U.S. Food and Drug Administration (FDA) has approved the Biotronik Orsiro drug-eluting stent ...
Clinical study data makes the world go around in cardiology and is the basis of setting guidelines in evidence-based ...

In recent years, there has been a lot of focus by vendors on developing better stenting technologies to treat peripheral ...

There was a lot of hype and high hopes pinned on bioresorbable stent technologies as the way of the future two years ago ...


 October 11, 2019
October 11, 2019