October 11, 2017 — An analysis of more than 1,000 minimally invasive aortic valve replacements and more than 400 ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
October 2, 2017 — Medtronic recently announced a new post-market clinical study to evaluate its CoreValve Evolut Pro ...
September 22, 2017 — The Montreal Heart Institute (MHI) announced the acquisition of the da Vinci Xi, a new-generation s ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
September 21, 2017 — Edwards Lifesciences Corp. recently received U.S. Food and Drug Administration (FDA) approval for ...
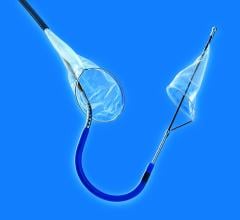
September 18, 2017 – Claret Medical announced publication of a new study in the Journal of the American College of ...
Ziyad Hijazi, M.D., MPH, MSCAI, FACC, director of the cardiac program and chair of the Department of Pediatrics at Sidra ...

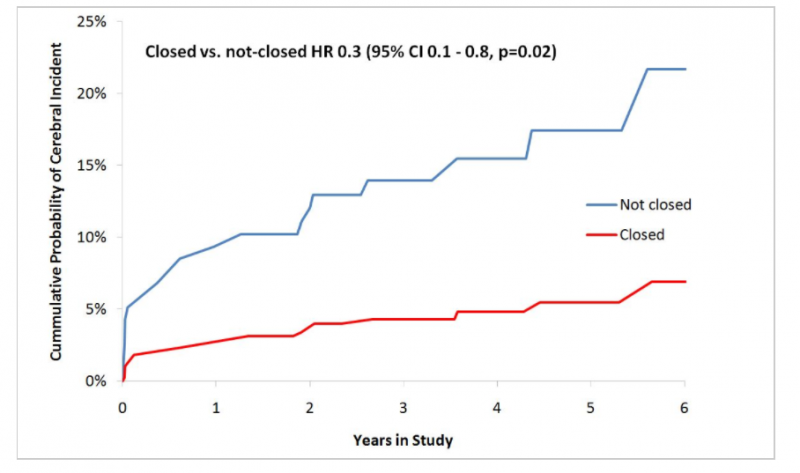
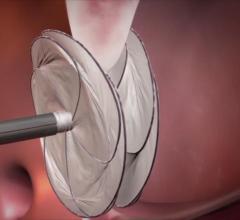
September 7, 2017 — Closure of the left atrial appendage (LAA) during heart surgery protects the brain, according to ...
September 7, 2017 — Protembis GmbH announced the first clinical applications of its ProtEmbo Cerebral Protection System ...
September 6, 2017 — The U.S. Food and Drug Administration (FDA) has cleared TrueFusion, a new cardiovascular application ...
September 5, 2017 — Minneapolis Heart Institute Foundation announced it has enrolled the first-in-the-world patient in a ...
September 5, 2017 — Abbott announced it has initiated a U.S. pivotal clinical study evaluating the safety and ...
August 31, 2017 — NaviGate Cardiac Structures Inc. (NCSI) announced that its Gate catheter-guided tricuspid ...
Azeem Latib, M.D., MBBCh, FCP, interventional cardiologist at Columbus Hospital in Milan, Italy, discusses the latest ...
August 23, 2017 — PinnacleHealth is the first hospital in Pennsylvania and one of the first 10 in the country to ...

 October 11, 2017
October 11, 2017