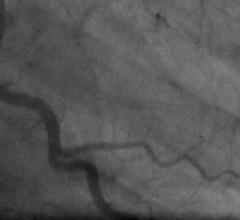
November 1, 2017 — The double kissing (DK) crush two-stent technique was associated with a lower rate of target lesion ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
November 1, 2017 — Analytics 4 Life announced it will be presenting new clinical data on the company's ongoing Coronary ...
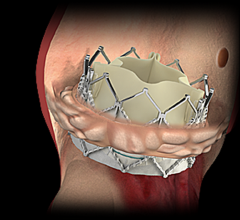
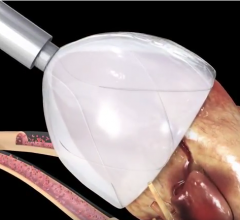
November 1, 2017 — Analysis of the PARTNER 2A trial and the SAPIEN-3 Intermediate Risk registry found transcatheter ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
November 1, 2017 — Results from the prospective, randomized, multicenter CULPRIT-SHOCK trial found an initial strategy ...
October 31, 2017 — The VAMPIRE 3 study evaluated if the selective use of distal filter protection might decrease the ...
October 31, 2017 — Edwards Lifesciences Corp. announced new data demonstrating substantial economic advantages of the ...
October 31, 2017 – Thirty-day results from ABSORB IV, the largest randomized everolimus-eluting bioresorbable vascular ...
October 31, 2017 — The U.S. Food and Drug Administration (FDA) issued a warning to healthcare providers that interim ...
October 30, 2017 — New study results from the EXCEL trial comparing the quality of life (QoL) of patients with left main ...
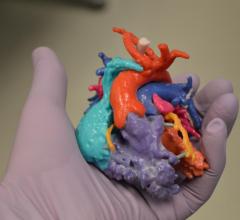
October 30, 2017 — Entering their pre-market phase of development, Materialise is working with select U.S. and EU ...
October 27, 2017 — CeloNova BioSciences Inc. announced the U.S. Food and Drug Administration (FDA) approved expansion of ...
Adam Greenbaum, M.D., co-director of the structural heart program at Henry Ford Health System in Detroit, discusses the ...

Here is an aggregation of DAIC news, videos and articles for recent heart valve technology advances in 2017.
TAVR ...
October 27, 2017 — CorInnova Inc. announced it has received notice of allowance of a seminal patent to protect its ...

Here is an aggregation of DAIC content for all major cardiovascular technology news and advances this past year. These ...

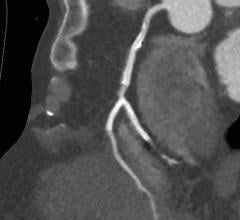
 November 01, 2017
November 01, 2017