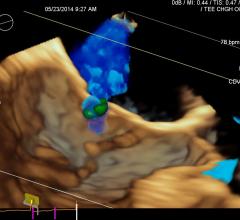
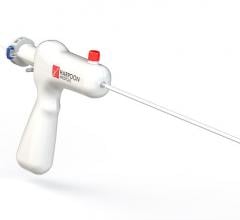
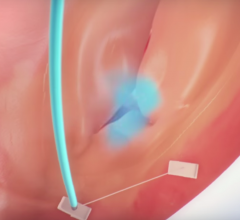
Ted Feldman, M.D., MSCAI FACC FESC, director of the cardiac cath lab, Evanston Hospital, explains the current state of ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
November 7, 2017 — Medtronic plc recently presented positive data for its self-expanding Intrepid transcatheter mitral ...
November 7, 2017 — A new study has found that a pioneering device to repair heart valves is safe and effective, and can ...
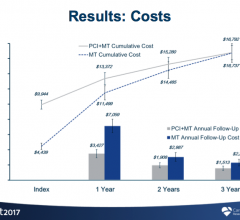
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
November 6, 2017 — In the Hybrid REvascularization Versus Standards (HREVS) study that evaluated three different ...
November 6, 2017 – Results from ORBITA, a prospective multi-center randomized blinded placebo-controlled study, found no ...
November 6, 2017 — One year follow-up data from the SCOUT I Early Feasibility Study for the Mitralign Trialign System ...
November 6, 2017 — The first trial to evaluate the safety of dual antiplatelet therapy (DAPT) for less than 12 months ...
November 6, 2017 — Three-year data from the FAME 2 study show patients with coronary artery disease who underwent a ...
November 6, 2017 — Elderly patients undergoing percutaneous coronary intervention (PCI) often receive bare-metal stents ...
November 3, 2017 — Biotronik's Orsiro drug-eluting stent (DES) demonstrated high long-term safety and clinical ...
November 3, 2017 — Acist Medical Systems Inc., a Bracco Group Company, announced the global launch of its Acist RXi Mini ...

November 2, 2017 – Five-year results from the PREVAIL Trial comparing left atrial appendage closure (LAAC) with the ...
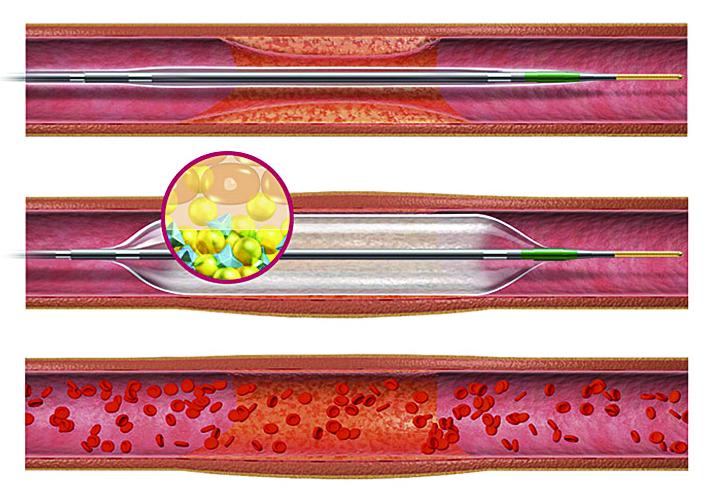
November 2, 2017 — The treatment of in-stent restenosis (ISR) remains challenging in clinical practice. The DARE (Drug ...
November 2, 2017 — Crossing the occlusion with a guidewire is often the most challenging part of chronic total occlusion ...
November 1, 2017 — OrbusNeich reported results from the REDUCE trial in the Late-Breaking Clinical Trial session at the ...

 November 07, 2017
November 07, 2017