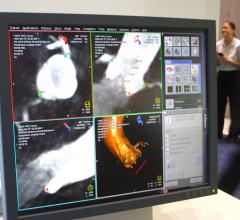
Juan Granada, M.D., executive director and chief scientific officer of the Cardiovascular Research Foundation's Skirball ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
Brijeshwar Maini, M.D., and Brian Bethea, M.D., from Tenet Florida’s structural heart program, explain the importance of ...
Krishna Rocha-Singh, M.D., Prairie Vascular Institute, Springfield, Ill., explains advancements in device therapy for ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
David Kandzari, M.D., director of interventional cardiology and chief scientific officer, Piedmont Heart Institute ...
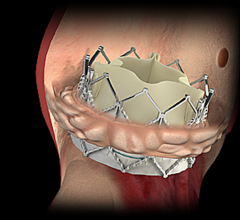
Studies have shown transcatheter aortic valve replacement (TAVR) has an increased risk of stroke and cerebral damage due ...
November 7, 2016 — Edwards Lifesciences Corp. recently announced new data demonstrating dramatic and sustained ...

November 7, 2016 – Results from the U.S. real-world, post-FDA approval experience of the Watchman device found high ...
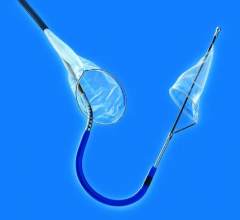
November 7, 2016 — Keystone Heart Ltd. announced that data presented at the Transcatheter Cardiovascular Therapeutics ...
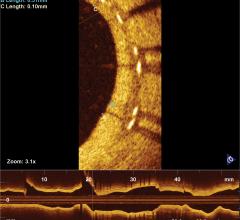
November 7, 2016 – Results from TRANSFORM-OCT, a prospective, randomized trial using optical coherence tomography (OCT) ...
November 7, 2016 – A multicenter randomized trial evaluating the role of embolic protection using the Sentinel device ...
November 7, 2016 – The two-year results from LEADERS FREE, the first randomized clinical trial dedicated to high ...
November 7, 2016 — The 28th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, Oct. 29-Nov. 3 ...
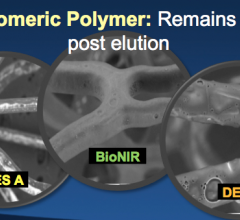
November 7, 2016 – The large multinational randomized BIONICS study found that a novel elastic polymer coated ...
November 7, 2016 – Two-year results from the COLOR Trial, the first large-scale multicenter prospective study of its ...
November 3, 2016 — Coronary artery bypass (CABG) surgery is the standard treatment for revascularization in patients ...


 November 09, 2016
November 09, 2016