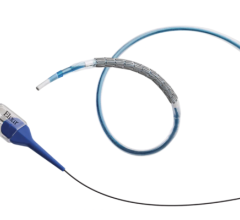
June 18, 2024 — Elixir Medical has announced the company’s novel bioadaptive implant, DynamX Sirolimus-Eluting Coronary ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
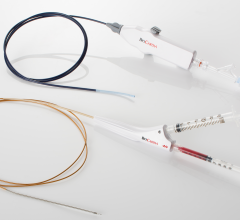
June 17, 2024 — Medtronic launched the Steerant Aortic Guidewire, tailored to facilitate catheter placement and exchange ...
June 13, 2024 — The U.S. Food and Drug Administration (FDA) announced that Teleflex, and their subsidiary Arrow ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 12, 2024 — Royal Philips, a global leader in health technology, announced the first implant of the Duo Venous Stent ...
June 7, 2024 — Access to thrombectomy should be expanded to include patients who experience basilar artery occlusion ...
June 7, 2024 — BioCardia, Inc., a company focused on cellular and cell-derived therapeutics for the treatment of ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
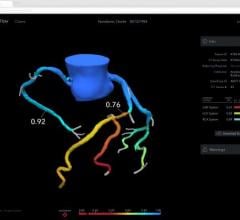
June 4, 2024 — HeartFlow, a leader in cardiovascular healthcare technology, is pleased to announce a key Medicare policy ...
June 4, 2024 — A patient at HonorHealth Research Institute is one of the nation’s first — and the first in Arizona and ...

Here is a look at the Top 10 pieces of content viewers were reading during the month of May.
1. American College of ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 3, 2024 — Elixir Medical, a developer of transformative technologies to treat cardiovascular and peripheral disease ...
May 31, 2024 — Johnson & Johnson announced it has completed its acquisition of Shockwave Medical. Shockwave is now part ...
May 30, 2024 — Biotronik announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 23, 2024 — HeartFlow, Inc., a leader in non-invasive artificial intelligence (AI) heart care solutions, announced ...
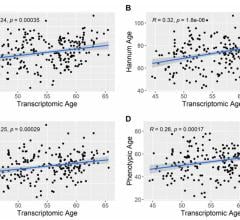
May 21, 2024 — A new research paper was published in Aging (listed by MEDLINE/PubMed as "Aging (Albany NY)" and "Aging ...
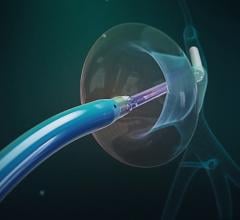
May 15, 2024 —CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac ...

 June 18, 2024
June 18, 2024