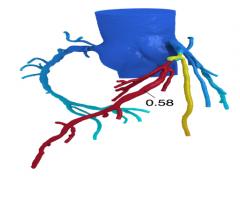
October 15, 2015 — Biotronik has announced results from the BIOSOLVE-II trial, investigating the safety and clinical ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
October 15, 2015 — C. R. Bard Inc. announced the presentation of the 12-month results from the Lutonix Global Real-World ...
October 14, 2015 — Tryton Medical Inc. announced results from the pivotal Tryton Confirmatory Study confirming the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
October 14, 2015 — On Oct. 7, 2015, Cook Medical initiated a voluntary recall for select sizes of Beacon Tip ...
October 9, 2015 — Cardinal Health is launching its expanded portfolio in the cardiovascular space including the latest ...
October 8, 2015 — Shimadzu Medical Systems USA announced the availability of the MIX package for the Trinias ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 6, 2015 – The results of a research study indicate that interventional cardiologists receive “very high” ...
October 5, 2015 — Beaumont Hospital - Royal Oak is the first in Michigan and one of just a handful in the United States ...
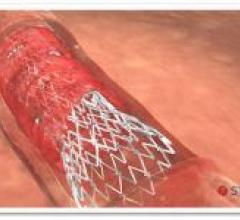
October 5, 2015 — The U.S. Food and Drug Administration (FDA) has approved Boston Scientific’s Synergy Bioabsorbable ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 2, 2015 — Tryton Medical Inc. announced that results of a post hoc analysis of the pivotal Tryton Randomized ...
October 2, 2015 — AstraZeneca announced that Brilinta (ticagrelor) 60-mg tablets are now available in U.S. pharmacies ...
October 1, 2015 — Intact Vascular Inc. announced the U.S. Food and Drug Administration has granted conditional approval ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 1, 2015 — Kimihiko Kichikawa, M.D., department of radiology at Nara Medical University in Japan, reported two ...
October 1, 2015 — Stentys announced that the company’s drug-eluting stent received CE Mark for treatment of below-the ...
October 1, 2015 — Galt Medical has partnered with Health Line International Corp. and launched the Nexus CT Midline Cath ...


 October 15, 2015
October 15, 2015