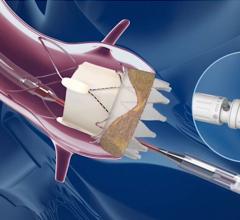
August 16, 2021 — The U.S. Food and Drug Administration (FDA) approved Abbott's Amplatzer Amulet Left Atrial Appendage ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
August 9, 2021 — Boston Scientific Corp. has commenced enrollment in the HI-PEITHO clinical trial, a collaborative ...
August 5, 2021 — The U.S. Food and Drug Administration (FDA) has cleared clearance Abbott's latest optical coherence ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
July 30, 2021 – Innovative Health Inc. announced several new initiatives to increase savings from single-use device ...
July 27, 2021 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation (BDD) to a laser ...
July 26, 2021 – CathWorks announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) has approved the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
July 22, 2021 — Medis Medical Imaging is partnering with CORRIB Core Lab and Sinomed in randomized clinical trial of ...
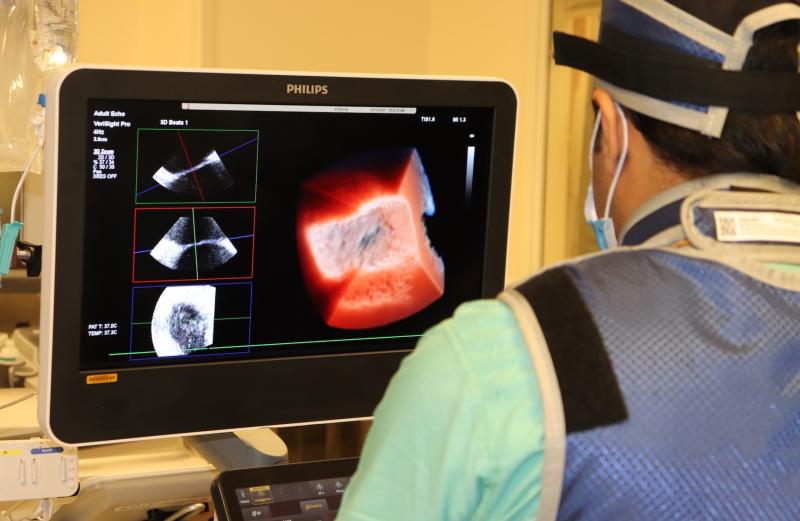
July 21, 2021 — Northwestern Medicine Bluhm Cardiovascular Institute recently became the first cardiovascular program in ...
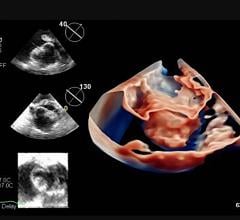
July 16, 2021 — Mayo Clinic recently became the first to use a next-generation 4-D intracardiac echo (ICE) imaging ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
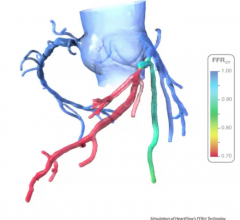
July 15, 2021 — HeartFlow, which has commercialized noninvasive computed tomography derived fractional flow reserve (FFR ...
July 15, 2021 — Vivasure Medical announced its development program for PerQseal Blue, a sutureless and fully ...
Doctor Andreas Ruck, interventional cardiologist and head of the mitral/tricuspid program, Karolinska University ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 13, 2021 — East End Medical announced it received U.S. Food and Drug Administration (FDA) clearance for the company ...
July 7, 2021 – Robocath, a company that designs, develops and commercializes cath lab robotic solutions to treat ...
July 1, 2021 — The U.S. Food and Drug Administration (FDA) has cleared the Angel Medical Systems Inc. second-generation ...


 August 16, 2021
August 16, 2021