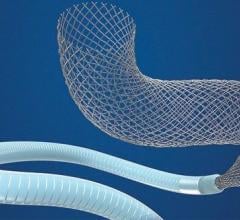
October 4, 2021 — U.S. Food and Drug Administration (FDA) has cleared the Abbott Amplatzer Talisman PFO Occlusion System ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
October 4, 2021 — One month of dual antiplatelet therapy (DAPT) following stent implantation in high bleeding risk ...
Tom Jones, M.D., director, cardiac cath labs, Seattle Children’s Hospital, and principle investigator of the Medtronic ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
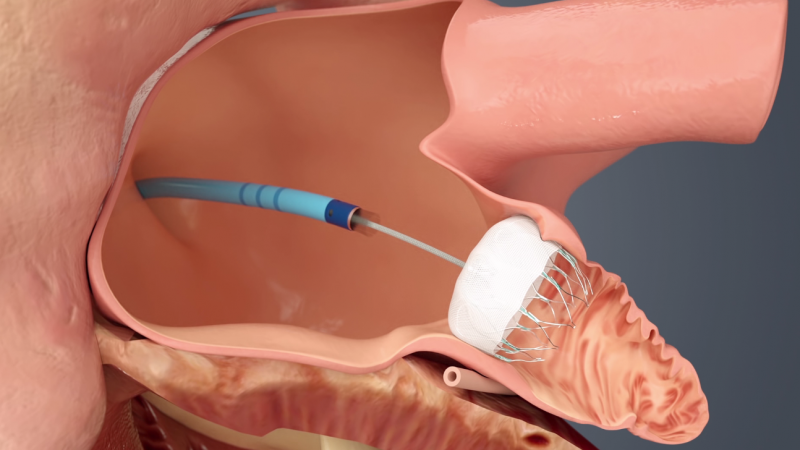
September 27, 2021 — Compared with men undergoing left atrial appendage occlusion (LAAO), women have a significantly ...
September 21, 2021 — Medtronic is recalling its Pipeline Flex Embolization Device and Pipeline Flex Embolization Device ...
Dr. Neil Moat, MBBS, chief medical officer of Abbott's structural heart business, explains the latest advances in Abbott ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
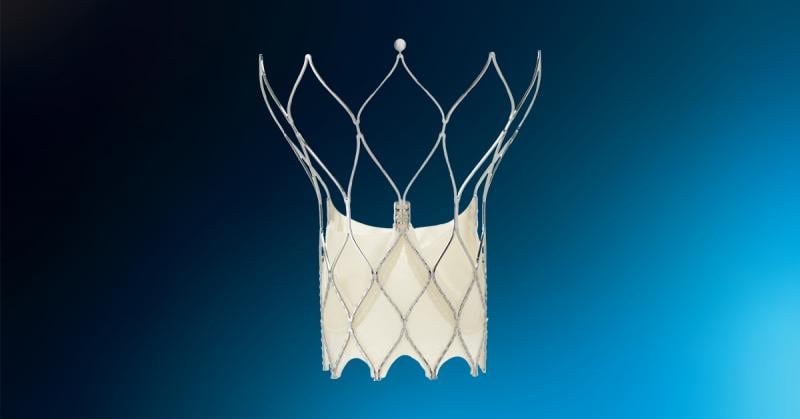
September 20, 2021 — Abbott announced that the U.S. Food and Drug Administration (FDA) has cleared the company's Portico ...
September 20, 2021 — The American Heart Association (AHA) announced Sept. 16 it decided to convert from a planned in ...
Tom Jones, M.D., director, cardiac cath labs, Seattle Children’s Hospital, explains the importance of the heart team ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

In the electrophysiology (EP) lab, hundreds of thousands of used devices are sent to reprocessors every year to get ...
September 13, 2021 — The European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery ...
September 13, 2021 — Early coronary angiography in out-of-hospital cardiac arrest (OHCA) patients without ST-segment ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
September 8, 2021 — Adding systematic fractional flow reserve (FFR) assessment to coronary angiography does not reduce ...
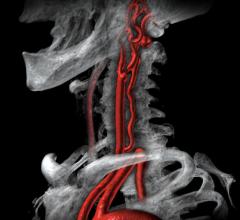
September 7, 2021 — Carotid artery surgery and stenting have comparable long-term effects on fatal or disabling stroke i ...
September 1, 2021 — The STOPDAPT-2 ACS trial does not support the use of one month of dual antiplatelet therapy (DAPT) ...


 October 04, 2021
October 04, 2021