November 7, 2019 — There was no difference between drug-coated balloons (DCB) vs. plain old balloon angioplasty (POBA) ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
November 6, 2019 — Cleveland Clinic announced the Top 10 Medical Innovations for 2020 at a multimedia presentation last ...

November 6, 2019 — Edwards Lifesciences announced it received European CE mark to expand use of the Edwards Sapien 3 ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

November 6, 2019 – The final, long-term, patient-level data for the Cook Medical Zilver PTX drug-eluting stent (DES) ...
November 6, 2019 – Positive, two-year data from the first-in-human study of Selution SLR, MedAlliance’s novel sirolimus ...
November 6, 2019 – Boston Scientific announced positive data for two of its devices within the peripheral drug-eluting ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

The latest in interventional cardiology clinical data and new device technologies were highlighted at the annual Transca ...
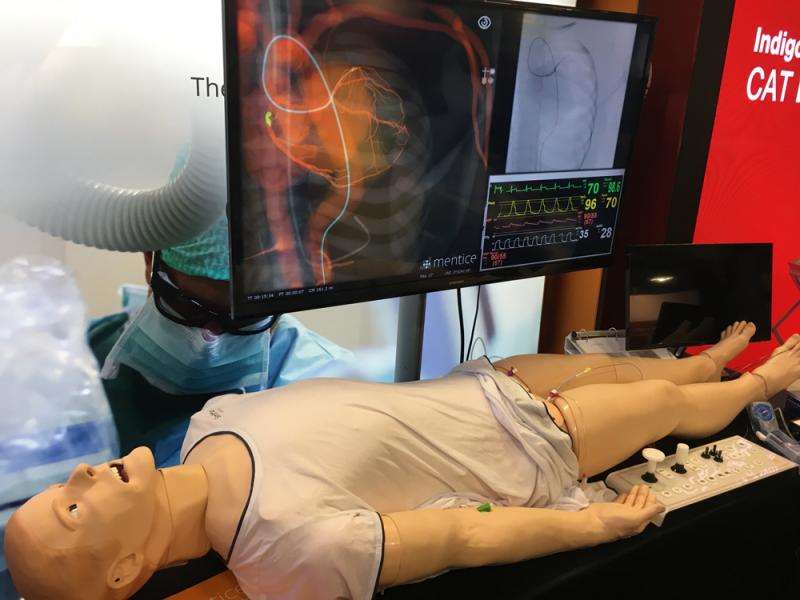
This is a photo essay of the interventional cardiology and structural heart technologies on the expo floor and discussed ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

November 1, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) m ...
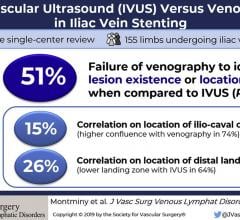
October 31, 2019 – In a large series of iliac vein stent cases, a blinded comparison found intravascular ultrasound ...
October 31, 2019 — Baylis Medical announced the first North American use of its EPstar Fixed Electrophysiology Catheters ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

October 29, 2019 — When Scripps cardiologists discovered early in 2019 that retired National Football League (NFL) great ...
October 29, 2019 — Siemens Healthineers completed the acquisition of 100 percent of Corindus Vascular Robotics Inc ...
Office based labs (OBLs) and ambulatory surgery centers (ASCs) require a fresh perspective from imaging vendors. The Alp ...


 November 07, 2019
November 07, 2019