Researchers at the National, Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH) ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

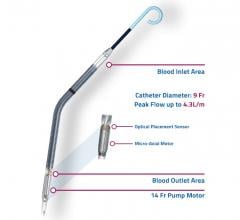
May 22, 2019 — Abiomed announced that the Impella CP with SmartAssist will be commercially available beginning at the 20 ...
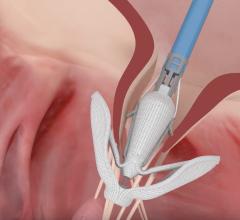
May 22, 2019 — Edwards Lifesciences Corp. announced strategic clinical and regulatory milestones for its Edwards Pascal ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
May 21, 2019 – Medtronic plc announced its entrance into the guide extension catheter market with the global launch of ...
May 20, 2019 – Researchers at the Icahn School of Medicine at Mount Sinai have demonstrated that stem cells derived from ...
May 20, 2019 — Philips announced the launch of the new IntraSight interventional applications platform. The secure ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
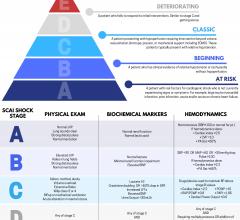
May 20, 2019 – A newly released expert consensus statement proposes a classification schema for cardiogenic shock (CS) t ...
This is a walk through of the primary structural heart hybrid cath lab at Henry Ford Hospital in Detroit, Mich. It is ...
A demonstration of how to calculate the neo-left ventricular outflow tract (neo-LVOT) on CT imaging for a transcatheter ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 17, 2019 — The U.S. Food and Drug Administration (FDA) this week approved Fragmin (dalteparin sodium) injection, for ...
May 17, 2019 — At the 40th annual Heart Rhythm Scientific Sessions, May 8-11 in San Francisco, Acutus Medical announced ...
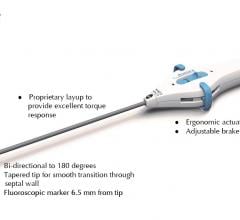
May 16, 2019 — BioCardia Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the Avance steerable ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
This is an example of a carotid artery reporting module from Change Healthcare at 2018 Radiological Society of North ...
May 15, 2019 — W. L. Gore & Associates Inc. (Gore) announced the U.S. Food and Drug Administration (FDA) has granted ...
May 15, 2019 — Cordis, a Cardinal Health company, recently announced the full U.S. launch of its Radial 360 portfolio ...

 May 22, 2019
May 22, 2019