Nevin Kapur, M.D., FAHA, FACC, FSCAI, executive director, Cardiovascular Center for Research and Innovation, Tufts ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
September 28, 2018 — Heart failure patients who received the Carillon Mitral Contour System in the REDUCE FMR clinical ...
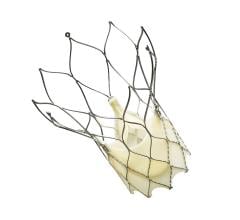
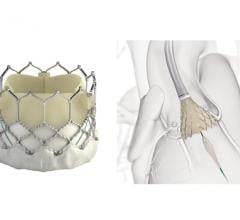
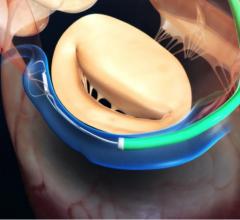
September 28, 2018 — At one year following transcatheter aortic valve replacement (TAVR), implantation with the Portico ...
September 28, 2018 — The first randomized study to compare general versus local anesthesia during transcatheter aortic ...
Philippe Genereux, M.D., co-director of the structural heart program at the Gagnon Cardiovascular Institute at ...
Akshay Khandelwal, M.D., director of medical operations at the Henry Ford Heart and Vascular Institute, Detroit, and ...
William Abraham, M.D., FACC, professor of medicine and director of the division of cardiovascular medicine, The Ohio ...
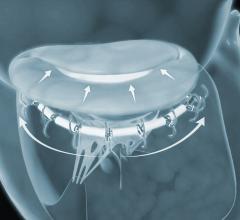
September 26, 2018 — Patients with heart failure and secondary mitral regurgitation (MR) who remained symptomatic ...
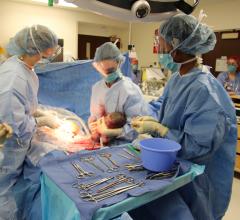
September 25, 2018 — Five-month-old Jack Palmer is home with his family in Kansas City after undergoing an extremely ...
September 24, 2018 — Ancora Heart Inc. announced positive clinical data from the company’s recently expanded U.S. early ...
September 24, 2018 — Getinge is voluntarily initiating a worldwide recall involving a field correction of approximately ...

October 4, 2018 – The Cardiovascular Research Foundation (CRF) had 15 late-breaking trials and 12 late-breaking clinical ...
September 20, 2018 — Cardiac Dimensions announced the company has randomized its first patient in the CARILLON Pivotal ...
September 19, 2018 — Abiomed Inc. announces its initiatives at the 30th Transcatheter Cardiovascular Therapeutics (TCT) ...
September 19, 2018 – The U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) ...


 October 01, 2018
October 01, 2018