May 10, 2016 — The first-ever trial using new SonR hemodynamic sensor technology proves to be safely and effectively ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
May 9, 2016 — Results from a first-of-its-kind study identify a significant increase in the incidence of atrial ...
May 9, 2016 — A new study shows the use of novel anticoagulants to treat atrial fibrillation (AF) on an “as needed basis ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 9, 2016 — New data presented at the Heart Rhythm Society's (HRS) annual meeting highlighted the long-term safety of ...
May 9, 2016 — A new study has found an increased risk of dementia in patients with atrial fibrillation (AF) that receive ...
May 9, 2016 —St. Jude Medical announced presentation of the MultiPoint Pacing (MPP) Investigational device exemption ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 9, 2016 — A new study presented at the Heart Rhythm Society’s 37th Annual Scientific Sessions, found that among ...
May 9, 2016 — Medtronic announced the results of several studies evaluating a novel approach to implantable cardioverter ...
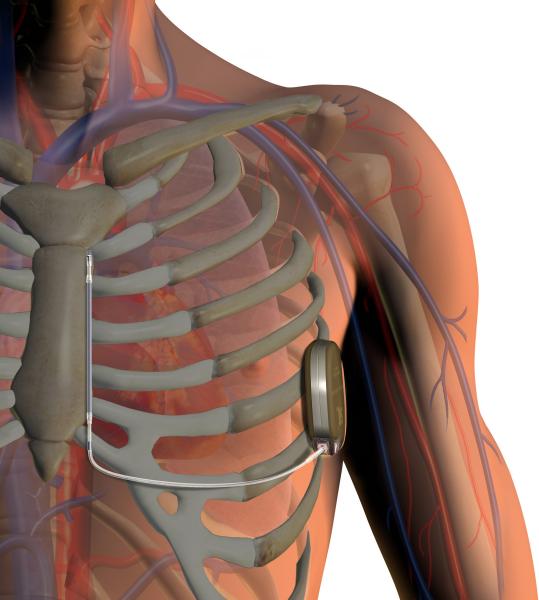
May 9, 2016 — The mid to long-term results from the EFFORTLESS study, the largest subcutaneous implantable cardioverter ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
May 9, 2016 – A new, large study shows that 95.7 percent of individual leads were successfully removed using a procedure ...
May 6, 2016 — Atrial fibrillation patients taking warfarin are at higher risk of developing kidney failure if ...
May 4, 2016 — Acutus Medical announced CE Mark approval for its AcQMap High Resolution Imaging and Mapping System and ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 3, 2016 — Biotronik announced U.S. Food and Drug Administration (FDA) approval of Iperia ProMRI HF-T, a cardiac ...
May 2, 2016 — In a recent case series, clinicians from The Heart Center at Nationwide Children’s Hospital and The Ohio ...
April 29, 2016 — AtriCure Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the AtriClip PRO2 ...


 May 10, 2016
May 10, 2016