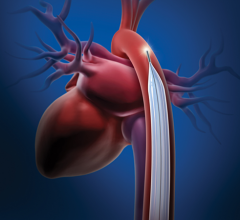
September 11, 2012 -- Abiomed Inc. announced it has received 510(k) clearance from the U.S. Food and Drug Administration ...
Hemodynamic Support Devices
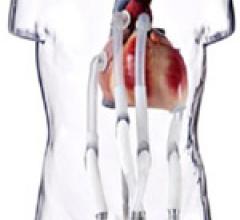
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.

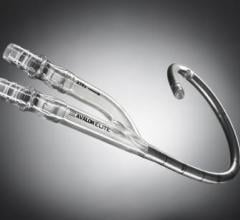
September 11, 2012 — Primary results from an ongoing trial by Maquet Cardiovascular using intra-aortic balloon pumps ...
September 10, 2012 — Maquet Cardiovascular LLC announced 30-day results from the large, randomized, multicenter intra ...
August 17, 2012 — Jarvik Heart Inc. announced U.S. Food and Drug Administration (FDA) approval of its pivotal trial for ...
August 14, 2012 — Berlin Heart announced that a study recently published in the New England Journal of Medicine (NEJM) ...
Aug. 8, 2012 — The Berlin Heart Group said the U.S. Food and Drug Administration (FDA) has granted approval for its post ...
June 14, 2012 — Abiomed Inc. said today it received Health Canada approval to market the Impella cVAD (ventricular ...
May 30, 2012 — MAQUET Cardiopulmonary, a business unit of MAQUET Cardiovascular and a leader in extracorporeal membrane ...
May 21, 2012 — Minnetronix announced the exclusive licensing rights of Penn State Research Foundation’s wireless energy ...
May 16, 2012 — Maquet Cardiovascular LLC announced this week that it has received 510(k) clearance from the U.S. Food ...
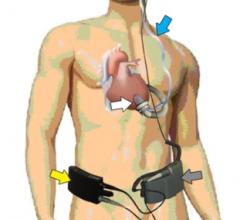
April 23, 2012 - CircuLite Inc. announced that updated clinical data related to the Synergy miniature ventricular ...
April 17, 2012 – The U.S. Food and Drug Administration (FDA) has approved a humanitarian use device (HUD) designation ...
April 12, 2012 - Abiomed Inc., a provider of heart support technologies, announced it has received CE marking approval ...
April 9, 2012 — Thoratec and the U.S. Food and Drug Adminstration (FDA) announced a recent Class 1 recall of the ...
March 26, 2012 — Maquet announced positive long-term mortality results from the Balloon Pump-Assisted Coronary ...


 September 11, 2012
September 11, 2012