September 6, 2023 — The Cardiovascular Research Foundation (CRF) has announced the TCT 2023 late-breaking clinical ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
June 26, 2023 — Building on the resounding success of last year’s standing-room-only debut, the Cardiovascular Research ...
December 30, 2022 — The Cardiovascular Research Foundation (CRF) recently announced that Alexandra Popma, MD, has joined ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
November 23, 2022 — The Cardiovascular Research Foundation (CRF) has announced its inaugural Pulse‑Setter Awards ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
At TCT 2022, Jane Leopold, MD, a cardiologist at Brigham and Women’s Hospital; Director, Women's Interventional ...
November 1, 2022 — Deepak L. Bhatt, MD, MPH, a top expert in cardiovascular medicine and interventional cardiology, has ...

Here is what you and your colleagues found to be most interesting in the fields of diagnostic and interventional ...
October 27, 2022 — Vascular surgeons from Allegheny General Hospital (AGH) Cardiovascular Institute, the flagship ...
At TCT 2022, Jane Leopold, MD, a cardiologist at Brigham and Women’s Hospital; Director, Women's Interventional ...
October 21, 2022 — Medtronic plc, a global leader in healthcare technology, today announced it has received U.S. Food ...
October 19, 2022 — Mount Sinai Health System’s globally acclaimed cardiologist Valentin Fuster, MD, PhD, has been named ...

October 17, 2022 — Significant findings from the highly-anticipated EPIC-STEMI study were presented during a late ...
At TCT 2022, Deepak Bhatt, MD, MPh, Executive Director of Interventional Cardiovascular Programs, Brigham and Women's ...
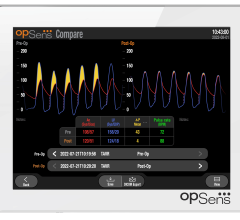
October 6, 2022 – OpSens Inc., a Quebec-based medical device cardiology-focused company, has announced that it has ...

 September 06, 2023
September 06, 2023