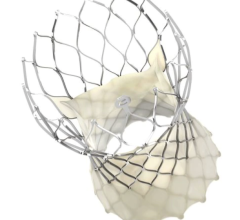
September 17, 2022 — Abbott today announced data from five late-breaking presentations showing the benefits of its ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
September 17, 2022 — Medtronic today announced findings from its Pathological Study of Hypo-Attenuated Leaflet ...
September 17, 2022 — Results of a new per-protocol analysis of the ST-segment Elevation Myocardial Infarction Door-To ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
September 17, 2022 — Boston Scientific Corporation has announced results from the PROTECTED TAVR clinical trial ...
September 16, 2022 — Edwards Lifesciences Corporation, today announced the company's PASCAL Precision transcatheter ...
September 16, 2022 — Teleflex Incorporated, a leading global provider of medical technologies, announced that Dr. Magnus ...
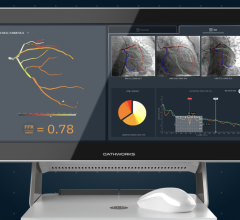
September 16, 2022 — CathWorks announced today the schedule of key events for the company during Cardiovascular Research ...
September 15, 2022 — The Cardiovascular Research Foundation (CRF) will honor Valentin Fuster, MD, PhD, Director of Mount ...
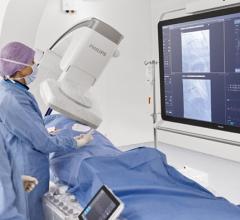
September 15, 2022 — Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, will showcase its ...

The Transcatheter Cardiovascular Therapeutics (TCT) conference, which is the Cardiovascular Research Foundation's (CRF) ...
September 12, 2022 — Boston Scientific Corporation has received U.S. Food and Drug Administration (FDA) approval to ...

Here is a rundown of what you and your colleagues found to be most interesting in the fields of diagnostic and ...
August 26, 2022 — The Cardiovascular Research Foundation (CRF) and Fogarty Innovation announced today that the program ...
It recently was announced that two leaders in cardiovascular disease science, research and education — the American ...
August 16, 2022 — Two leaders in cardiovascular disease science, research and education, the American Heart Association ...


 September 17, 2022
September 17, 2022