October 25, 2023 — For its novel multi-lumen stent system for interventional adjustment of pulmonary blood flow in ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
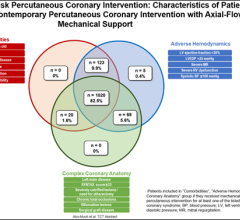
October 24, 2023 — Four years ago, following a publication based on the limited data available, the U.S. Food and Drug ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
October 23, 2023 — Supira Medical, Inc., a Shifamed portfolio company, announced that the company's percutaneous ...
October 20, 2023 — JenaValve Technology, Inc., developer and manufacturer of the Trilogy Transcatheter Heart Valve (THV) ...
October 20, 2023 — Over the coming days, Philips will be presenting its latest solutions in cardiology and new late ...

Innovation. Connection. Inspiration. The Transcatheter Cardiovascular Therapeutics (TCT) 2023 conference, the Cardiovas ...
October 19, 2023 — The Dib UltraNav Transseptal Catheter System, which houses a needle and ultrasound in one system for ...
October 19, 2023 — Conformal Medical, Inc. announced today the one-year results from the company's CONFORMAL Early ...
October 19, 2023 — Edwards Lifesciences Corporation announced the company's EVOQUE tricuspid valve replacement system ...
October 19, 2023 — OpSens, a medical device cardiology-focused company, has announced that results of the “SAFE-TAVI ...

October 18, 2023 — The following is an updated program preview of the Cardiovascular Research Foundation's (CRF) annual ...
October 10, 2023 — Elixir Medical, a developer of innovative cardiovascular technologies, announced it will present ...
October 10, 2023 — The recipients of two prestigious awards — the Career Achievement Award and the TCT Geoffrey O ...

September 26, 2023 — The Cardiovascular Research Foundation's (CRF) annual scientific symposium, the Transcatheter ...


 October 25, 2023
October 25, 2023