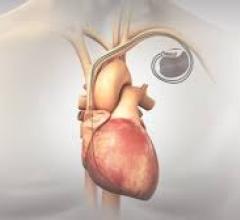
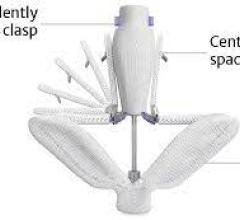
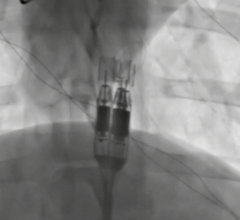
At TCT 2022, Dr. Akl Fahed, Physician-scientist and Interventional Cardiologist, Massachusetts General Hospital and Harv ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.

The 34th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research ...

Here is a recap of what DAIC viewers found most interesting during the month of September:
1. Unloading with Impella ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...

The Photo Gallery includes images taken by DAIC's editorial team at the 34th Transcatheter Cardiovascular Therapeutics ( ...
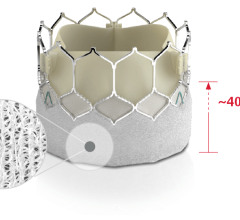
September 28, 2022 — In the "TCT Innovation" session Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator ...
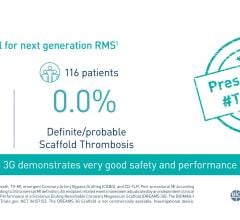
September 27, 2022 — BIOTRONIK announced the presentation of new full-cohort 2-year BIOSOLVE-IV data. In a poster ...
September 26, 2022 — Edwards Lifesciences announced the launch of the SAPIEN 3 Ultra RESILIA valve, which incorporates ...
September 26, 2022 — ReCor Medical, Inc., a wholly owned subsidiary of Otsuka Medical Devices Co., Ltd., announced the ...
September 22, 2022 — Abiomed (ABMD) announces the result of a three-year, investigator-led study of all Impella ...
September 22, 2022 — The recipient of the TCT 2022 Thomas J. Linnemeier “Spirit of Interventional Cardiology” Young ...
September 20, 2022 — Orchestra BioMed, Inc., a biomedical innovation company accelerating high-impact technologies to ...
September 20, 2022 — Results of the first randomized controlled trial to directly compare two contemporary transcatheter ...
September 20, 2022 — An analysis from the Society of Thoracic Surgeons/American College of Cardiology(STS/ACC) TVT ...
September 19, 2022 — Puzzle Medical Devices, a provider of hemodynamic support in Heart Failure (HF), was selected as ...
September 19, 2022 — A first-in-human (FIH) study using the ModulHeart device (Puzzle Medical Devices Inc.) has ...


 October 06, 2022
October 06, 2022