August 4, 2022 — The Cardiovascular Research Foundation (CRF) has announced the late-breaking clinical science that will ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
August 2, 2022 — Two significant awards will be presented at TCT 2022, to be held in Boston, Sept. 16-19, 2022.
Caree ...
July 29, 2022 — ReCor Medical, Inc. and Otsuka Medical Devices Co., Ltd., a fully owned subsidiary of Otsuka Holdings ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
July 12, 2022 — The Cardiovascular Research Foundation (CRF) and Fogarty Innovation announced today that they are ...
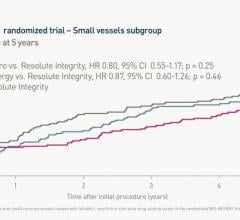
July 5, 2022 — A BIO-RESORT subgroup analysis of outcomes in small coronary vessels (<2,5mm) evaluated the efficacy and ...
Dr. Simon Dixon, MBChB, chair of the Department of Cardiovascular Medicine at Beaumont Hospital Royal Oak, the Dorothy ...
Srihari Naidu, M.D., FACC, FAHA, FSCAI, is the director of both the cardiac cath labs and Hypertrophic Cardiomyopathy ...
November 12, 2021 — Penumbra Inc. announced that the CHEETAH clinical study of its Indigo System CAT RX Catheter ...
November 12, 2021 — Orchestra BioMed Inc. announced multiple presentations of long-term clinical results and ISH ...
November 10, 2021 — Medtronic presented early data for its self-expanding Intrepid transcatheter mitral valve ...
November 10, 2021 — Biosensors International Group Ltd., a developer and manufacturer of innovative medical devices ...
November 10, 2021 — Shockwave Medical a pioneer in the development of Intravascular Lithotripsy (IVL) to treat severely ...
November 10, 2021 — Ancora Heart Inc., a company developing a novel therapy to address heart failure, announced results ...
November 10, 2021 — Philips Healthcare announced North American availability of new innovations in its portfolio of ...
November 10, 2021 — There were nine late-breaking trials and 13 late-breaking science presentations at the 2021 Transcat ...

 August 04, 2022
August 04, 2022