September 12, 2017 – The Cardiovascular Research Foundation (CRF) announced the promotion of Juan F. Granada, M.D., as ...
September 12, 2017 – The European Society of Cardiology (ESC) Congress 2017 includes several Hot Line Late-breaking ...
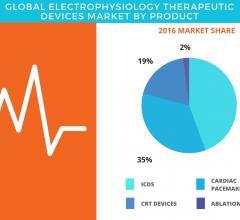
September 11, 2017 — According to the latest market study released by Technavio, the global electrophysiology ...
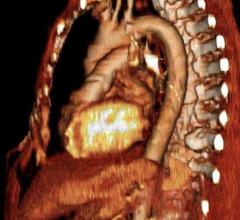
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Merck and researchers in the Clinical Trial Service Unit at the University of Oxford announced the publication and presentation of results from the REVEAL outcomes study of anacetrapib, Merck’s investigational cholesteryl ester transfer protein (CETP) inhibitor. In the study of 30,449 patients with atherosclerotic vascular disease receiving LDL-C lowering treatment with atorvastatin, anacetrapib significantly reduced the risk of major coronary events (composite of coronary death, myocardial infarction or coronary revascularization) by 9 percent relative to placebo (10.8% vs. 11.8%, respectively; P=0.004). The safety of anacetrapib was generally consistent with data from earlier trials of the drug. However, a sub-study also showed that anacetrapib accumulates in adipose tissue with prolonged dosing.
September 11, 2017 — Veniti Inc. announced that Boston Scientific will distribute the Vici Venous Stent under a limited ...
September 11, 2017 — Researchers have led a retrospective single-center study examining simple hemodynamic parameters ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A cardiovascular service line manager reader of DAIC recently e-mailed me and asked if I had a list of the top ...
September 8, 2017 — ClearFlow Inc. announced recently that positive clinical trial results were presented at the ...

September 8, 2017 — Abbott Vascular has announced it will end commercial sales of its Absorb bioresorbable vascular ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 7, 2017 — Sapheneia and Scannerside received U.S. Food and Drug Administration (FDA) 510(k) clearance to ...
The U.S. Food and Drug Administration (FDA) announced it would hold a meeting of the Medical Imaging Drugs Advisory Committee (MIDAC) on Sept. 8 to discuss regulatory approaches for use of gadolinium-based contrast agents (GBCAs).
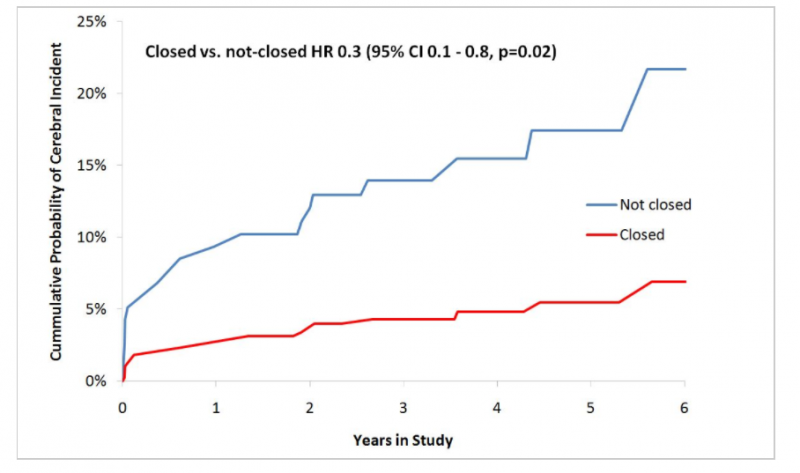
September 7, 2017 — Closure of the left atrial appendage (LAA) during heart surgery protects the brain, according to ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 7, 2017 — Oxygen therapy does not improve survival in patients with heart attack symptoms, according to late ...

September 7, 2017 — Researchers at the European Society of Cardiology (ESC) Congress called for a reconsideration of ...
In July, The Heart Institute at Florida Medical Center became the first hospital in the state of Florida to offer the U.S. Food and Drug Administration (FDA)-cleared high sensitive Elecsys Troponin T Gen 5 blood test (Troponin T Gen 5 or TnT Gen 5) to aid in the diagnosis of heart attacks. The new nine-minute test offers faster answers clinicians can use when diagnosing heart attack.

 September 12, 2017
September 12, 2017