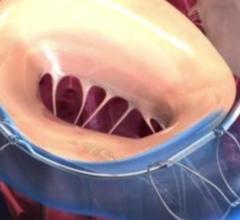
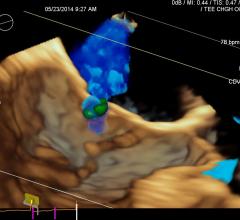
November 7, 2017 – A new study has found that a pioneering device to repair heart valves is safe and effective, and can ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

Ted Feldman, M.D., MSCAI FACC FESC, director of the cardiac cath lab, Evanston Hospital, explains the current state of ...
November 7, 2017 — Medtronic plc recently presented positive data for its self-expanding Intrepid transcatheter mitral ...
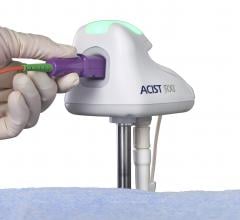
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
November 7, 2017 — A new study has found that a pioneering device to repair heart valves is safe and effective, and can ...
November 6, 2017 — In the Hybrid REvascularization Versus Standards (HREVS) study that evaluated three different ...
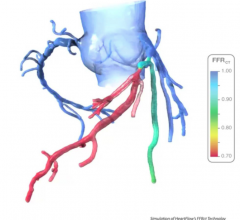
November 6, 2017 – Results from ORBITA, a prospective multi-center randomized blinded placebo-controlled study, found no ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
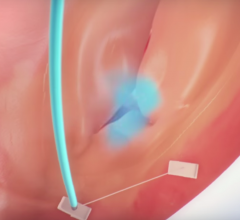
November 6, 2017 — One year follow-up data from the SCOUT I Early Feasibility Study for the Mitralign Trialign System ...
November 6, 2017 — The first trial to evaluate the safety of dual antiplatelet therapy (DAPT) for less than 12 months ...
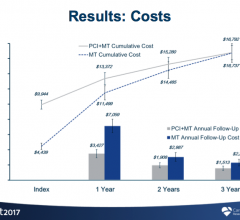
November 6, 2017 — Three-year data from the FAME 2 study show patients with coronary artery disease who underwent a ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 6, 2017 — Elderly patients undergoing percutaneous coronary intervention (PCI) often receive bare-metal stents ...
November 6, 2017 – The Centers for Medicare & Medicaid Services (CMS) has finalized a New Technology Ambulatory Payment ...
November 3, 2017 — Biotronik's Orsiro drug-eluting stent (DES) demonstrated high long-term safety and clinical ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

November 3, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional ...
November 3, 2017 — Acist Medical Systems Inc., a Bracco Group Company, announced the global launch of its Acist RXi Mini ...

November 2, 2017 – Five-year results from the PREVAIL Trial comparing left atrial appendage closure (LAAC) with the ...

 November 07, 2017
November 07, 2017