Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
January 27, 2023 — The first US patient has been enrolled at Medstar Washington Hospital Center in the SELUTION4ISR ...
January 25, 2023 — According to a new release from the U.S. Food and Drug Administration (FDA), Datascope, a subsidiary ...
January 13, 2023 — A change to Medicare policy surrounding heart transplant may lead to increased inequities in access ...
January 12, 2023 — Selution SLR, MedAlliance’s novel sirolimus-eluting balloon, has received conditional FDA ...
December 28 2022 — Abiomed announces the United States Food and Drug Administration (FDA) has approved the version of Im ...
December 22, 2022 — Johnson & Johnson today announced it has completed its acquisition of Abiomed, Inc. Abiomed is now ...

The U.S. Food and Drug Administration (FDA) has issued a Class I recall on the Arrow AutoCAT 2 and AC3 Intra-Aortic ...
November 30, 2022 — The Smidt Heart Institute at Cedars-Sinai has selected board-certified cardiothoracic surgeon Tyler ...
November 15, 2022 — Ten months after transplanting the first genetically-modified pig heart into a human patient ...
November 7, 2022 — The immediate use of veno-arterial mechanical circulatory extracorporeal membrane oxygenation (ECMO) ...
November 2, 2022 — For decades, left ventricular-assist devices (LVADs) have extended the lives of people whose hearts ...
November 1, 2022 — Abiomed announced that Impella RP Flex with SmartAssist has received U.S. Food and Drug ...
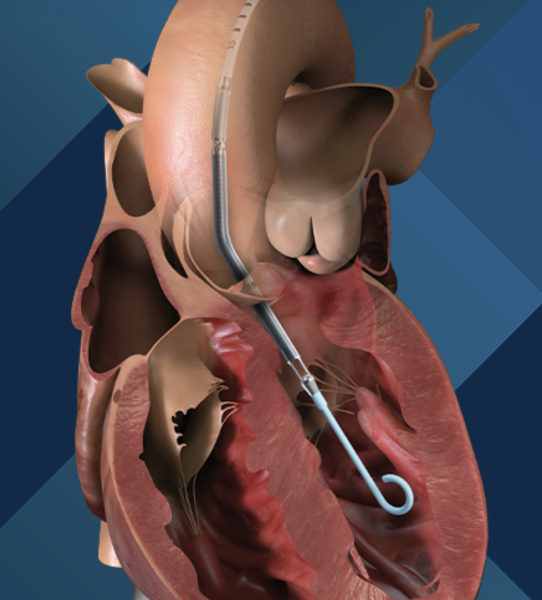
November 1, 2022 — Johnson & Johnson and Abiomed, a world leader in breakthrough heart, lung and kidney support ...
October 26, 2022 — Abiomed (Nasdaq: ABMD) announces a new program to address healthcare disparities in underserved ...


 February 24, 2023
February 24, 2023