May 13, 2022 — Vascular diseases are public enemy number one: the leading killers worldwide, accounting for nearly a ...
Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
April 28, 2022 — The U.S. Food and Drug Administration (FDA) is alerting healthcare providers to the possibility that ...
March 28, 2022 — Carmat, the designer and developer of the world’s most advanced total artificial heart, aiming to ...

The University of Maryland Medical Center has announced that David Bennett, the 57-year old patient with terminal heart ...
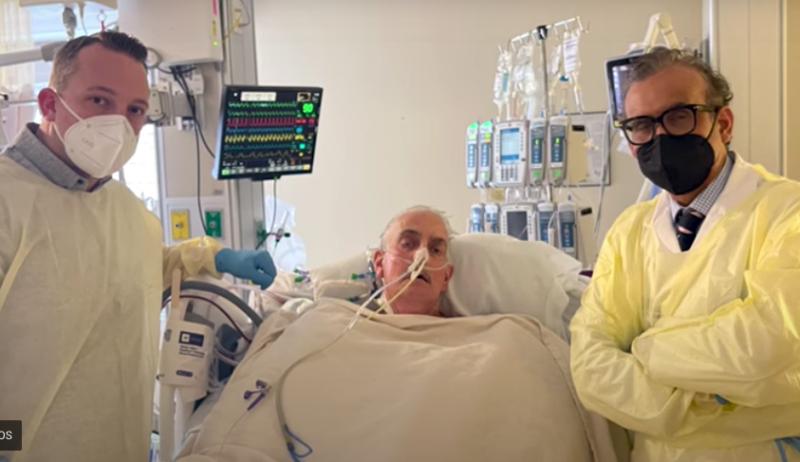
In January, The University of Maryland School of Medicine (UMSOM) and the University of Maryland Medical Center (UMMC) ...
February 9, 2022 — Philips, a global leader in health technology, announced it has expanded its ultrasound portfolio wit ...
February 8, 2022 — Extracorporeal membrane oxygenation (ECMO) is a lifesaving treatment for critically ill neonates ...
The University of Maryland Medical Center (UMMC) announced the first successful transplant of a genetically modified pig ...

January 10, 2022 — The University of Maryland School of Medicine (UMSOM) and the University of Maryland Medical Center ...
December 26, 2021 — Here is a list of the most popular videos on the DAIC website from the past year in 2021 based on ...
December 16, 2021 — Datascope/Getinge/Maquet has recalled the Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic ...
December 7, 2021 – Critically ill patients with COVID-19 can safely be transported for lifesaving extracorporeal ...
Srihari Naidu, M.D., FACC, FAHA, FSCAI, is the director of both the cardiac cath labs and Hypertrophic Cardiomyopathy ...
November 2, 2021 — Datascope/Getinge/Maquet is recalling its Cardiosave Hybrid/Rescue Intra-Aortic Balloon Pumps (IABP) ...
October 20, 2021 – CorWave, a French medtech company developing a next-generation heart pump, won the 2021 HealthTech ...


 May 13, 2022
May 13, 2022

![With the addition of Pulse Wave Doppler [1], Philips has greatly expanded the utility of its Handheld Ultrasound – Lumify – enabling clinicians to quantify blood flow in a wide range of point-of-care diagnostic applications – including cardiology, vascular, abdominal, urology, obstetrics and gynecology.](/sites/default/files/styles/content_feed_medium/public/Screen%20Shot%202022-02-09%20at%209.23.01%20AM.png?itok=sb5JOa7n)