June 7, 2024 — Calcific aortic valve disease (CAVD) is the major heart valve disease that afflicts nearly 10 million ...
Cardiovascular Clinical Studies
This channel includes news and new technology innovations from cardiovascular clinical trials. These clinical studies include all cardiac subspecialties.
June 7, 2024 — BioCardia, Inc., a company focused on cellular and cell-derived therapeutics for the treatment of ...
June 7, 2024 — Medtronic today announced new data from the CoreValve Evolut Clinical Program, reinforcing the positive ...

June 7, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of the ...
June 6, 2024 —Cleveland Clinic researchers found higher amounts of the sugar alcohol xylitol are associated with ...
June 5, 2024 — Lexicon Pharmaceuticals, Inc. (Nasdaq: LXRX) today announced a new post-hoc analysis of clinical data ...
June 4, 2024 — Caristo Diagnostics Limited, a global provider of cardiovascular disease diagnostics and risk prediction ...
June 3, 2024 — Elixir Medical, a developer of transformative technologies to treat cardiovascular and peripheral disease ...
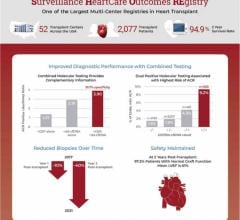
May 31, 2024 — CareDx, a leading precision medicine company focused on the discovery, development, and commercialization ...
May 30, 2024 — Elixir Medical, a developer of technologies to treat cardiovascular and peripheral disease, announced ...
May 30, 2024 — Biotronik announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC ...
May 29, 2024 — Data was presented by AstraZeneca on AZD0780, an oral PCSK9 inhibitor which demonstrated significant LDL ...
May 24, 2024 — Lexicon Pharmaceuticals, Inc. announced a new post-hoc analysis of clinical data showing that INPEFA ...
May 23, 2024 — HeartFlow, Inc., a leader in non-invasive artificial intelligence (AI) heart care solutions, announced ...
May 23, 2024 — Global Heart Hub, the international alliance of heart patient organizations, announced the first findings ...

 June 07, 2024
June 07, 2024