Here is a compilation of some of DAIC's recent video coverage of new interventional cardiology device technologies.
...
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
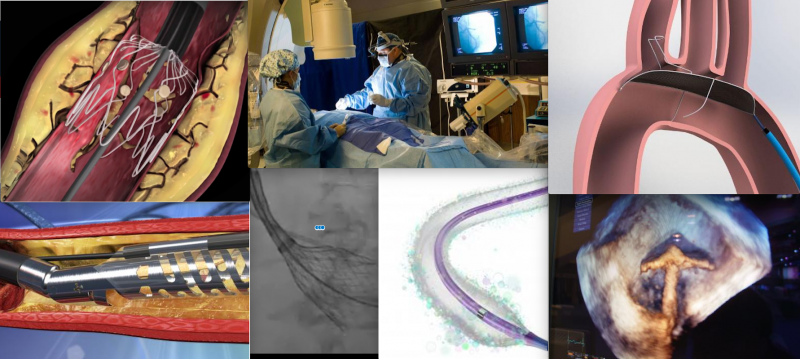
Here is a compilation of some of DAIC's recent video coverage of new interventional cardiology device technologies.
...
This video, provided by Tryton, demonstrates the implantation of the Tryton Side Branch Stent. It became the first ...
March 6, 2017 — ON Semiconductor announced the release of a new CCD image sensor that enables video imaging under ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
March 6, 2017 – The U.S. Food and Drug Administration (FDA) has granted pre-market approval (PMA) for the Tryton Side ...
March 3, 2017 — Biosensors International Group Ltd. announced in February enrollment of the first patient in LEADERS ...

March 3, 2017 — A Henry Ford Hospital study found a 100 percent success rate in left atrial appendage (LAA) occlusion ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
March 3, 2017 — In a United Kingdom patent case involving a Edwards Lifesciences lawsuit against Boston Scientific for ...
March 3, 2017 — Philips recently announced the global launch of Azurion, its next-generation image-guided therapy platfo ...
March 2, 2017 — BioVentrix Inc. announced that InEk, the German Institute for the Hospital Remuneration System, has ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
March 2, 2017 — Teleflex Inc. has announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U.S ...
March 2, 2017 — Intact Vascular Inc. announced in February that its Tack Optimized Balloon Angioplasty II Below the Knee ...
March 2, 2017 — Here is the list of the top 20 most popular pieces of content on the Diagnostic and Interventional ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
March 2, 2017 — The U.S. Food and Drug Administration (FDA) cleared CeloNova BioSciences Inc. first-in-class Cobra PzF ...
March 1, 2017 — BTG plc announced U.S. Food and Drug Administration (FDA) 510(k) clearance has been granted to the Ekos ...
February 28, 2017 — A U.S. Food and Drug Administration (FDA) panel has recommended market clearance for the Claret ...