July 17, 2015 - Hospitals in the Midwest were more likely than others to refer patients for guideline-recommended cardia ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).


July 17, 2015 - Building teams that include advanced practice providers can help cardiovascular practices meet the ...
July 16, 2015 — Medtronic plc announced that its Protege GPS self-expanding peripheral stent system has received ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
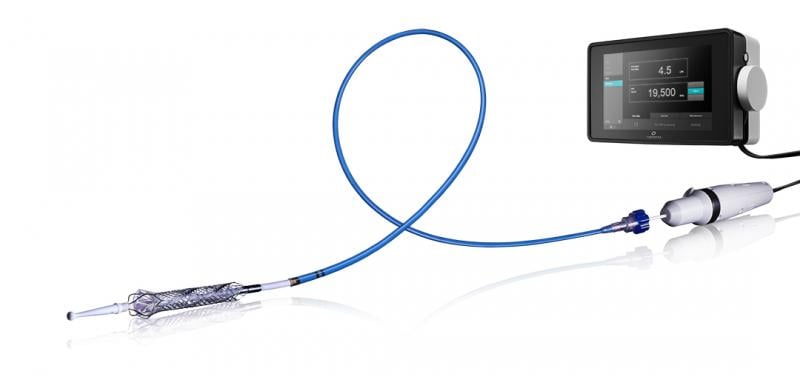
July 15, 2015 - Thoratec announced that the HeartMate PHP (Percutaneous Heart Pump) received CE Mark approval ...

July 13, 2015 - Hospitals performing carotid artery stenting vary considerably in rates of in-hospital stroke or death ...

July 13, 2015 - Edwards Lifesciences Corp. announced that it has agreed to acquire CardiAQ Valve Technologies Inc., a ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
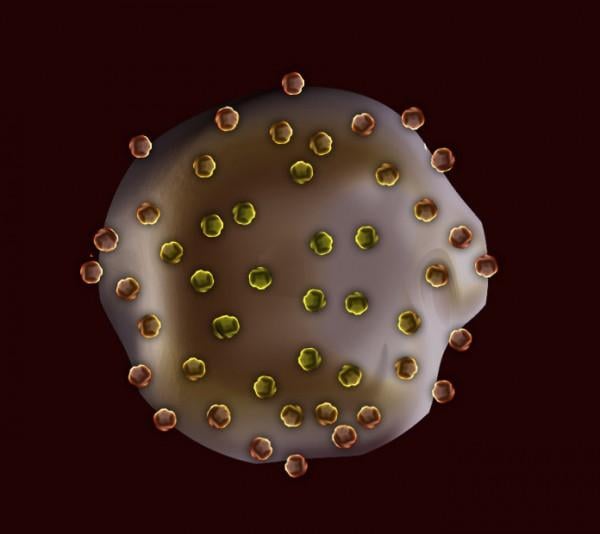
July 13, 2015 - Researchers have created tiny gel particles that can perform the same essential functions as platelets ...
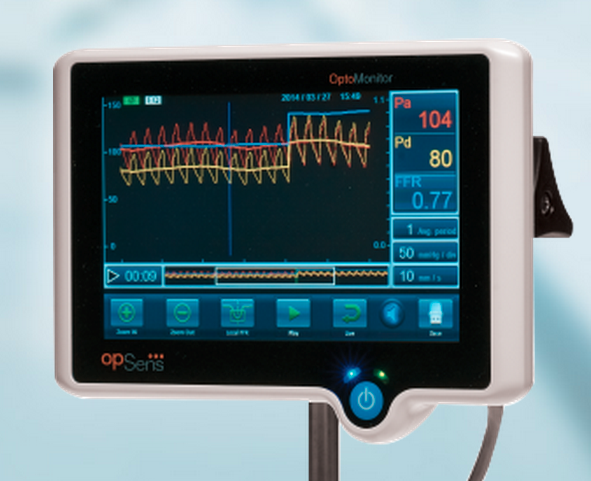
July 10, 2015 - Opsens Inc. announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the OptoWire ...

July 9, 2015 - St. Jude Medical, Inc. announced the launch of the ILUMIEN III clinical trial on June 30. ILUMIEN III is ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
July 9, 2015 - During an event with Massachusetts Gov. Charlie Baker in June, Abiomed announced a major expansion of its ...

July 8, 2015 - C.R. Bard Inc. announced the publication of results from the LEVANT 2 study in the June 24, 2015, online ...
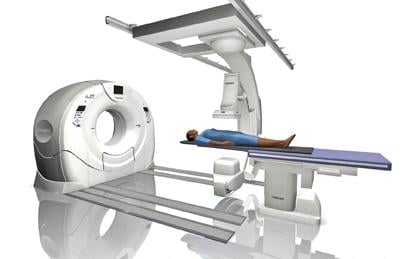
Angiographic imaging system vendors have developed several new technologies to address emerging cath lab trends ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 6, 2015 - DC Devices Inc. announced that it has completed enrollment and implants in the REDUCE LAP-HF trial, an ...
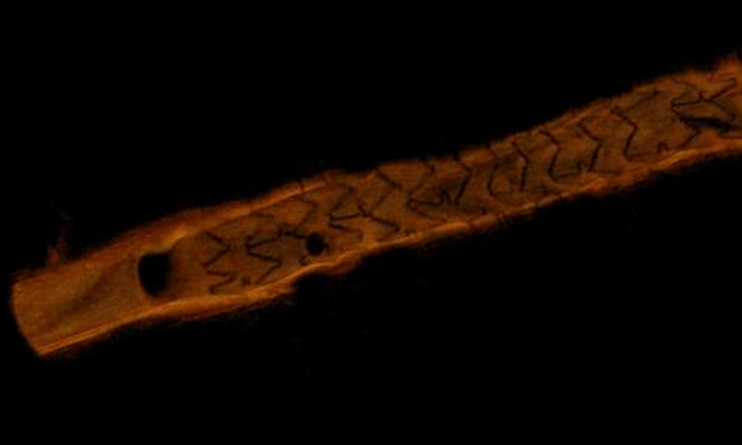
Angiography alone for procedural image guidance in the catheterization lab is sometimes not enough, and it is helpful to ...
Key to ongoing U.S. healthcare reform are efforts to reduce costs by eliminating unnecessary medical procedures and ...

 July 17, 2015
July 17, 2015






