March 1, 2018 – Patients who were treated for breast cancer or lymphoma are more than three times at risk for ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
February 28, 2018 — The American College of Cardiology’s Cardiovascular Summit will begin on Thursday, Feb. 22, 2018 ...
February 27, 2018 – A new study published in the Journal of the American College of Cardiology confirms non-invasive car ...
February 23, 2018 — Boston Scientific announced In November 2017 a delay to timelines for commercialization of the Lotus ...
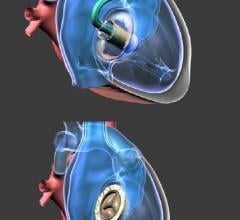
February 22, 2018 — NaviGate Cardiac Structures Inc. (NCSI)announced that on Feb. 2, 2018, its catheter-guided Gate valv ...
This video details the first use of a new protocol at The Ohio State University’s Wexner Medical Center to start sudden ...
February 22, 2018 — When someone goes into cardiac arrest and first responders cannot shock their heart back into rhythm ...
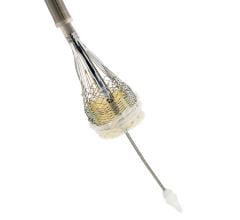
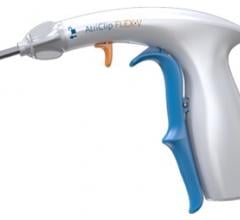
February 22, 2018 — AtriCure Inc. announced that it has launched the AtriClip FLEX•V Left Atrial Appendage (LAA) ...
February 21, 2018 — The U.S. Food and Drug Administration (FDA) issued the final rule on “Human Subject Protection ...
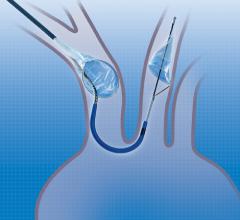
February 19, 2018 — Claret Medical announced that since U.S. Food and Drug Administration (FDA) clearance in June 2017 ...
February 16, 2018 — California’s Petaluma Health Center (PHC) was recently awarded a 2017 Healthcare Information and ...
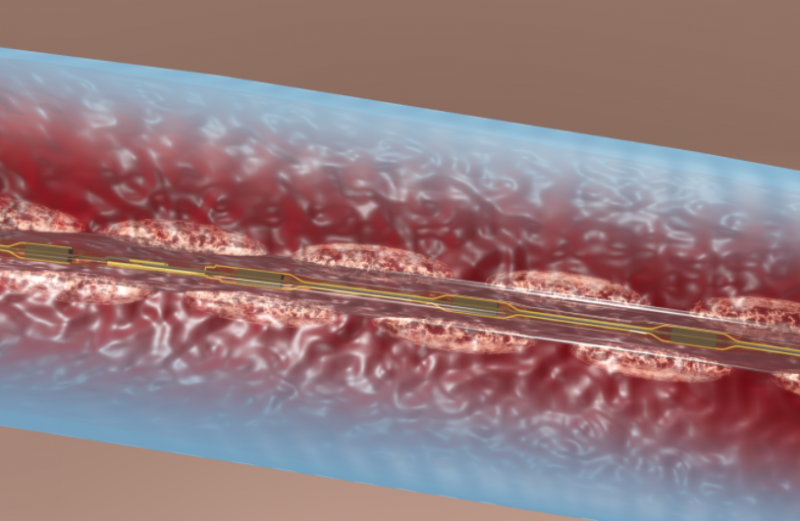
There are few downsides to using tibial venous access to treat deep vein thrombosis (DVT) with catheter-directed ...
February 15, 2018 — Abiomed Inc. announced that it received an expanded U.S. Food and Drug Administration (FDA) Pre ...
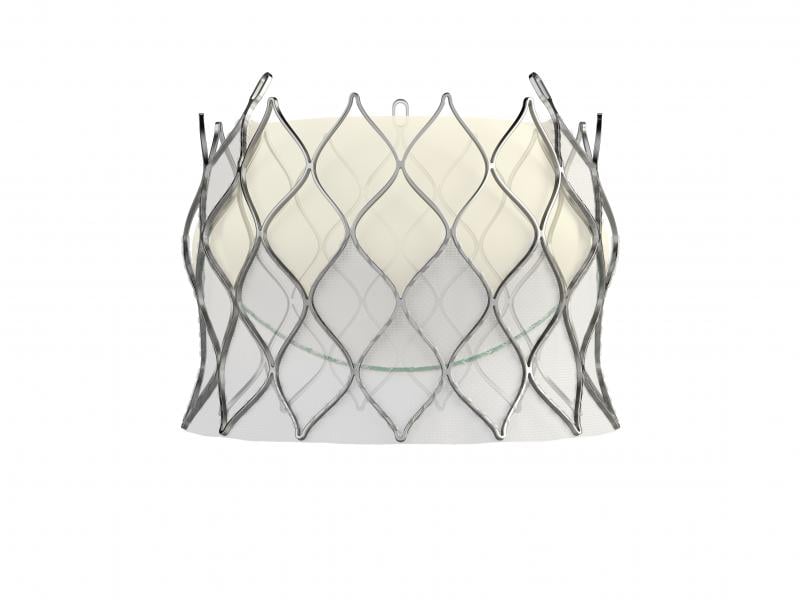
February 15, 2018 — Edwards Lifesciences Corp. has received European CE mark clearance for its self-expanding Centera ...
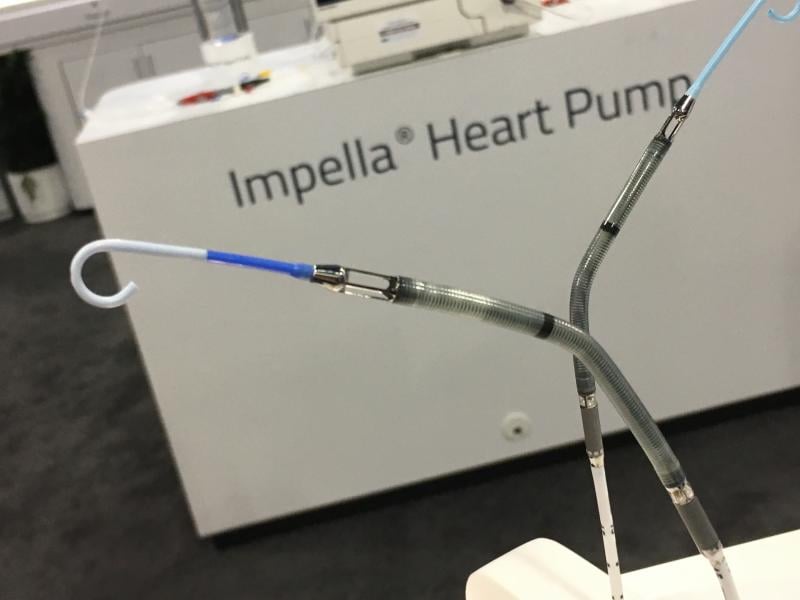
February 14, 2018 — Abiomed Inc. announced it received an expanded U.S. Food and Drug Administration (FDA) pre-market ...

 March 01, 2018
March 01, 2018