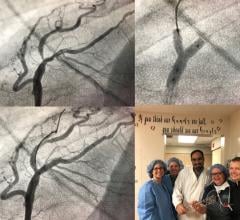
May 29, 2018 – Medtronic plc announced the initiation of a U.S. clinical study to assess the safety and efficacy of drug ...
Stents
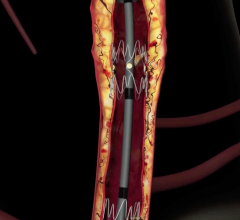
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
May 25, 2018 — Abbott announced it received approval from the U.S. Food and Drug Administration (FDA) for Xience Sierra ...
May 15, 2018 — The Society for Cardiovascular Angiography and Interventions (SCAI) has released new guidelines to ...
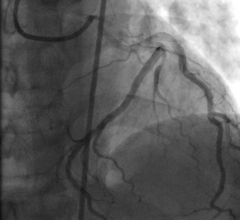
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 15, 2018 — A contemporary, real-world analysis shows lower mortality rates when culprit-only intervention is used ...
April 27, 2018 — Intact Vascular Inc. recently closed a Series C financing totaling $20 million. This financing is ...
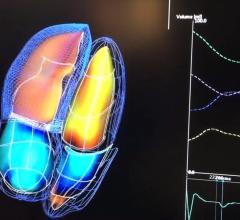
DAIC Editor Dave Fornell takes a tour of some of the most interesting new technologies on the expo floor at the 2018 ...
February 26, 2018 – Designed specifically for small vessels, Medtronic plc announced U.S. Food and Drug Administration ...
February 19, 2018 — The Detroit Medical Center’s (DMC) interventional cardiology team at Heart Hospital recently became ...
February 13, 2018 — Abbott announced the first patient has been enrolled in a clinical trial evaluating 28 days of dual ...
February 1, 2018 – Thomas Zeller (Bad Krozingen, Germany) presented the 12-month results from Veryan Medical’s MIMICS-2 ...
January 25, 2018 – Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling ...
January 18, 2018 – West Hills Hospital & Medical Center, a full-service acute care facility, announces the first ...
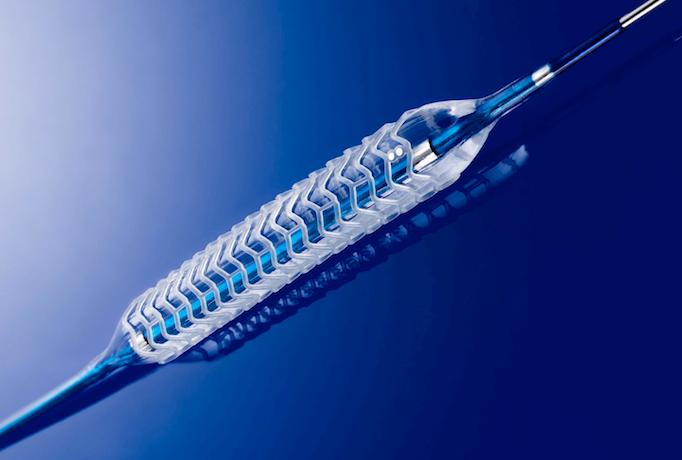
There was no shortage of interest in bioresorbable stent technologies at the many sessions offered on this topic or ...
December 14, 2017 — The U.S. Food and Drug Administration (FDA) announced clearance for NuMed’s Cheatham Platinum (CP) S ...


 May 29, 2018
May 29, 2018