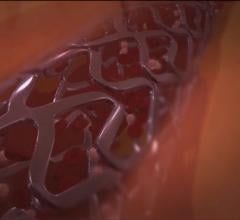
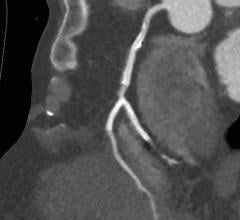
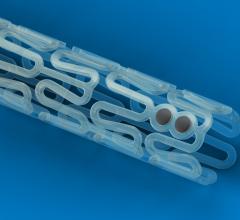
December 13, 2017 — Cordis, a Cardinal Health company, and Medinol recently announced U.S. Food and Drug Administration ...
Stents
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
November 24, 2017 – During a late-breaking session at the European Society of Cardiology (ESC) 2017 meeting, presented ...
Philippe Genereux, M.D., co-director of the structural heart program at the Gagnon Cardiovascular Institute at ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 10, 2017 — Cordis, a Cardinal Health company, recently unveiled a comprehensive interventional cardiology portf ...
November 9, 2017 — Abbott received European CE mark for Xience Sierra, the newest generation of the company's Xience ...

November 8, 2017 – New results from the HARMONEE Japan/U.S. Registration Trial, reported by in a first report ...
A discussion with Ajay Kirtane, M.D., SM, director of the cardiac catheterization laboratories at New York-Presbyterian ...
November 6, 2017 — The first trial to evaluate the safety of dual antiplatelet therapy (DAPT) for less than 12 months ...
November 6, 2017 — Elderly patients undergoing percutaneous coronary intervention (PCI) often receive bare-metal stents ...
November 3, 2017 — Biotronik's Orsiro drug-eluting stent (DES) demonstrated high long-term safety and clinical ...
November 1, 2017 — OrbusNeich reported results from the REDUCE trial in the Late-Breaking Clinical Trial session at the ...
November 1, 2017 — The double kissing (DK) crush two-stent technique was associated with a lower rate of target lesion ...
October 31, 2017 – Thirty-day results from ABSORB IV, the largest randomized everolimus-eluting bioresorbable vascular ...
October 31, 2017 — The U.S. Food and Drug Administration (FDA) issued a warning to healthcare providers that interim ...
October 27, 2017 — CeloNova BioSciences Inc. announced the U.S. Food and Drug Administration (FDA) approved expansion of ...

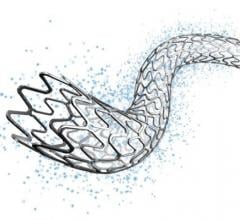
 December 13, 2017
December 13, 2017