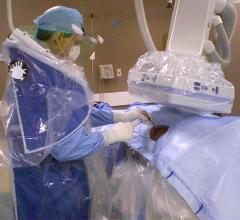
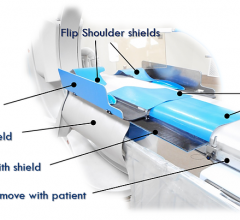
May 10, 2018 — A new consensus document has been issued to guide the optimal use of ionizing radiation in cardiovascular ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
In the past, there was not much that could be done to mitigate the X-ray radiation exposure of interventional ...
We started our design from the ground up, tested many prototypes, did not want to compromise on radiation protection for ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
May 9, 2018 — Infinitt North America announced that Infinitt Cardiology Suite-v 1.0.8.1 has achieved certification from ...
May 9, 2018 — The U.S. Food and Drug Administration (FDA) said it recently granted market clearance for Angel Medical ...
May 8, 2018 — Biosense Webster Inc. announced its Carto Vizigo Bi-directional Guiding Sheath is now available in the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
May 8, 2018 — One-year results from the Sentinel Cerebral Protection System show it can reduce the incidence of stroke d ...

The European interventional cardiology market is currently valued at nearly $1.4 billion. This is a mature market that ...
Imran Ahmad, M.D., medical director of interventional cardiology, explains some of the new technologies his labs have ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 7, 2018 — The U.S. Food and Drug Administration (FDA) has approved Portola Pharmaceuticals' Andexxa, the first ...

May 4, 2018 — Transcatheter valve technology has been advancing very quickly and the links to aggregated content from ...
May 3, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for the Medtronic In.Pact Admiral ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 2, 2018 — Conavi Medical Inc. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its ...
May 1, 2018 — Edwards Lifesciences Corp.announced that it received CE Mark for the Edwards Cardioband Tricuspid Valve ...

Here is the list of the most popular articles and videos on the Diagnostic and Interventional Cardiology (DAIC) magazine ...

 May 10, 2018
May 10, 2018