The introduction of thermal ablation revolutionized the treatment of varicose veins, yet recurrence remains a stubborn ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
April 27, 2018 — Intact Vascular Inc. recently closed a Series C financing totaling $20 million. This financing is ...
April 25, 2018 — HeartFlow Inc. announced that the National Health Service (NHS) England has chosen the HeartFlow FFRct ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
April 20, 2018 — Emerging medical device company Emboline Inc. announced it has completed a Series B funding round ...

While there is fear by many about the trend of hospitals consolidating into larger healthcare systems, one advantage ...
A 360 degree view of the newest cath lab at Northwestern Medicine Central DuPage Hospital in Winfield, Ill., located in ...
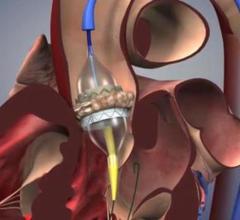
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Patient selection for who is best suited for a transcatheter aortic valve replacement (TAVR) procedure is partly based ...
April 18, 2018 — Many of the world’s leading interventional cardiologists and cardiovascular professionals will convene ...
Northwestern Medicine has purchased several smaller Chicago suburban hospitals in the past few years to expand its ...
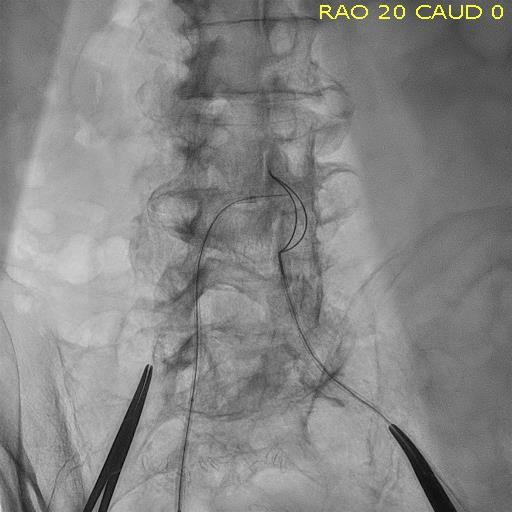
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
April 17, 2018 — The U.S. Food and Drug Administration (FDA) announced market approval for the Abbott Perclose ProGlide ...
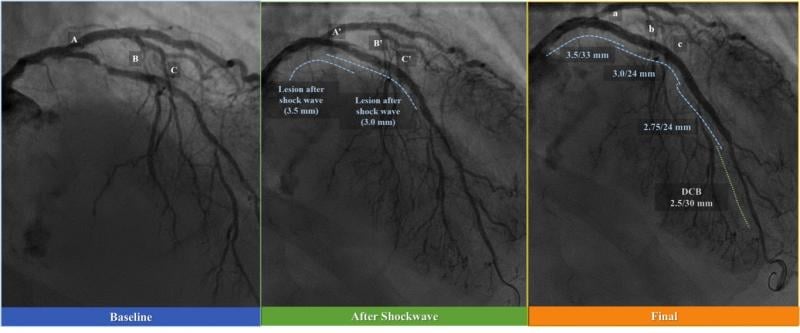
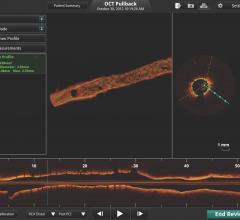
Over the last decade, there have been considerable developments in procedural techniques and technology facilitating the ...
April 16, 2018 — Medtronic plc announced U.S. Food and Drug Administration (FDA) approval to begin an investigational ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
The biggest workplace concern for interventional cardiologists and cath lab staff is their daily exposure to ...
April 11, 2018 — Abbott today announced the initiation of a clinical trial evaluating long-term outcomes of patients who ...
April 10, 2018 – Cardiva Medical announced the company received U.S. Food and Drug Administration (FDA) approval for an ...


 April 30, 2018
April 30, 2018