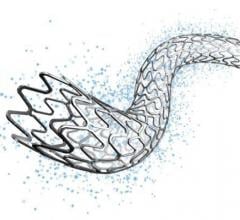
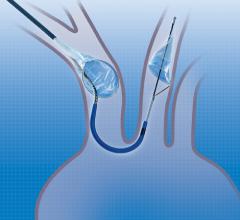
February 23, 2018 — CorFlow Therapeutics AG announced that the company will present new insights into the coronary ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
This video details the first use of a new protocol at The Ohio State University’s Wexner Medical Center to start sudden ...

Interventional cardiology has witnessed a rapid and constant evolution in both techniques and device technology since ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
February 22, 2018 — When someone goes into cardiac arrest and first responders cannot shock their heart back into rhythm ...
February 21, 2018 – Vascular closure device provider Cardiva Medical announced that the company has closed on $11 ...
February 20, 2018 — Corindus Vascular Robotics Inc. announced today that it received 510(k) clearance from the U.S. Food ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
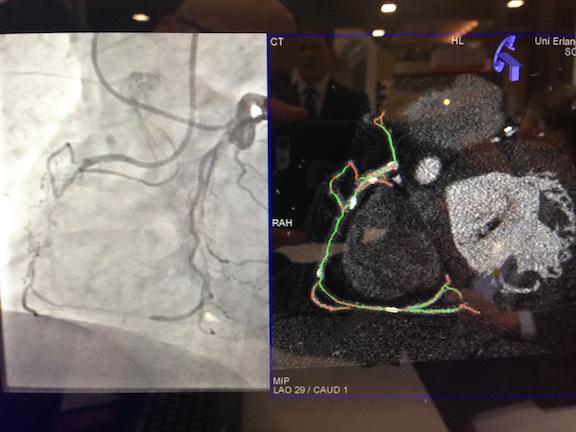
One of the recent hot topics in interventional cardiology has been how operators can use new tools and techniques to ...
February 19, 2018 — The Detroit Medical Center’s (DMC) interventional cardiology team at Heart Hospital recently became ...
February 19, 2018 — Claret Medical announced that since U.S. Food and Drug Administration (FDA) clearance in June 2017 ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
The father of transradial artery access, Ferdinand Kiemeneij, M.D., Ph.D., interventional cardiologist, The Netherlands ...
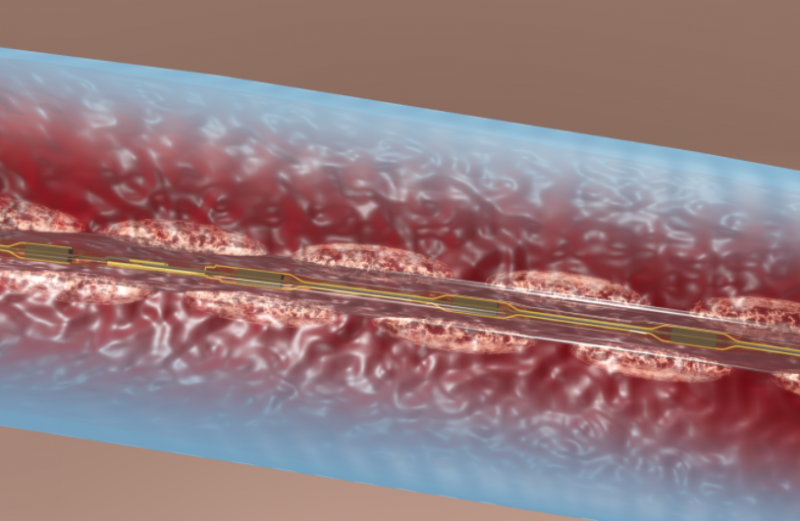
There are few downsides to using tibial venous access to treat deep vein thrombosis (DVT) with catheter-directed ...
February 15, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for Stryker's Trevo ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
February 15, 2018 — Abiomed Inc. announced that it received an expanded U.S. Food and Drug Administration (FDA) Pre ...
February 15, 2018 — The National Institutes of Health (NIH) awarded a $2.2 million research grant to healthcare ...
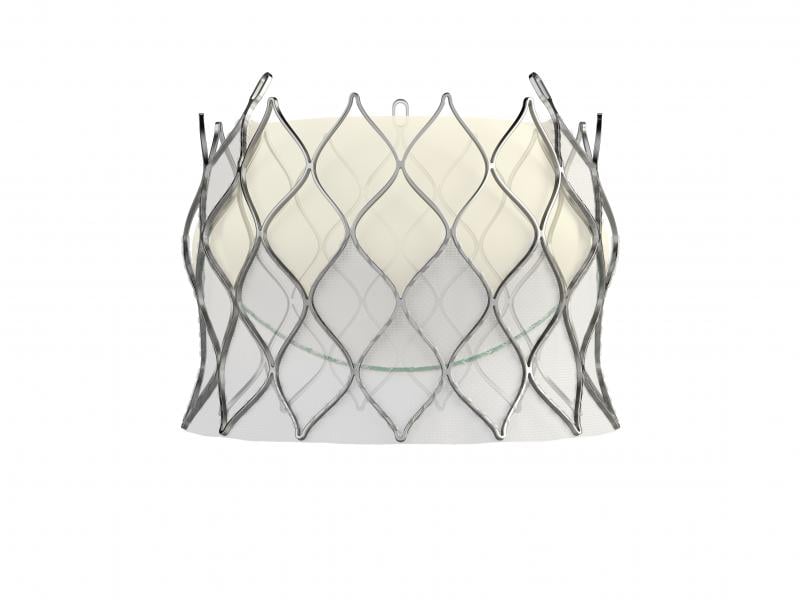
February 15, 2018 — Edwards Lifesciences Corp. has received European CE mark clearance for its self-expanding Centera ...

 February 23, 2018
February 23, 2018