August 30, 2023 — BIOFLOW-DAPT one-year-data demonstrated non-inferiority and a good safety profile for the Orsiro ...
Stents
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
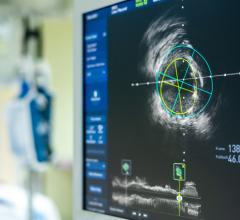
August 29, 2023 — Optical coherence tomography (OCT) is non-inferior to intravascular ultrasound (IVUS) for guiding ...
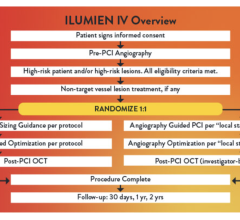
August 29, 2023 — Abbott announced late-breaking data from the first-of-its-kind ILUMIEN IV OPTIMAL PCI (ILUMIEN IV) ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
August 28, 2023 — Prasugrel monotherapy after percutaneous coronary intervention (PCI) with drug-eluting stents is not ...
August 22, 2023 — Heart attack patients who do not take daily aspirin have an elevated likelihood of recurrent myocardia ...
August 3, 2023 — Merit Medical Systems, a leading global manufacturer and marketer of healthcare technology, today ...
July 27, 2023 — Abiomed is recalling the Impella Intravascular Left Sided Blood Pumps because the pump’s Instructions ...
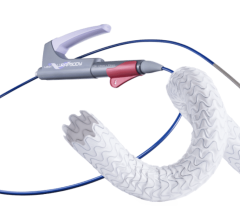
July 13, 2023 — Elixir Medical, a developer of breakthrough cardiovascular technologies, announced enrollment completion ...
July 12, 2023 — In a late breaking trial session during EuroPCR 2023 in Paris, on behalf of the HOST-IDEA study ...
June 26, 2023 — InspireMD, Inc., developer of the CGuard Embolic Prevention Stent System (EPS) for the prevention of str ...
June 22, 2023 — The Society of Interventional Radiology (SIR) published a new position statement offering ...
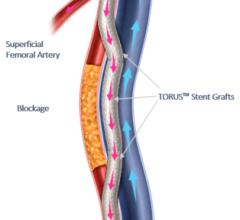
June 16, 2023 — Endologix LLC, a privately held, global medical device company, dedicated to providing disruptive ...
June 15, 2023 — Endologix LLC, a privately held, global medical device company dedicated to providing disruptive ...
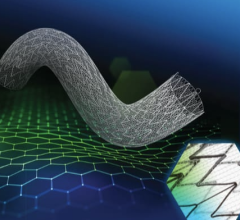
May 25, 2023 — First-generation bioresorbable vascular scaffolds (BVS) may be just as effective as drug-eluting metallic ...


 August 30, 2023
August 30, 2023