Behnood Bikdeli M.D., a cardiologist at the Brigham and Women’s Hospital, Harvard Medical School, Boston, offers an overview of numerous trials in progress testing a variety of anticoagulants and antiplatelet agents at different doses for inpatient, outpatient and severely ill ICU, and long-hauler COVID-19 (SARS-CoV-2) patients. Bikdeli is a cardiologist at the Brigham and Women’s Hospital, Harvard Medical School, Boston, and is involved in the Clinical Trials Center, Cardiovascular Research Foundation, New York, and Center for Outcomes Research and Evaluation (CORE), Yale School of Medicine, New Haven, Conn.
He is a the principal investigator for a comprehensive overview of clinical trials looking at anti-thrombotic drug therapies and dosing in coronavirus patients. The article "Recent Randomized Trials of Antithrombotic Therapy for Patients With COVID-19: JACC State-of-the-Art Review" was published online March 16, 2021.
Bikdeli gives an overview of more than 70 studies that are underway or recently completed. He also discussed the need to tailor therapy to specific patients, since there is no "one-size-fits all" approach.
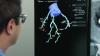
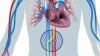
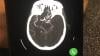
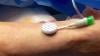
COVID causes thromboembolitic events in patients, including deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, heart attacks, and clots that can cause ischemia or infarcts in various organs. While consensus statements on how to treat or protect patients from COVID clotting were published last year, they were based on opinions, not on hard, clinical evidence. The current studies are hoped to offer answers on how to prophylax specific types of COVID patients in the next couple months.
Bikdeli also served as lead author on the INSPIRATION Randomized Clinical Trial, which was published in March and looked at the effect of intermediate-dose vs. standard-dose prophylactic anticoagulation for COVID patients on extracorporeal membrane oxygenation (ECMO). He discusses the results of this study as well in the video.
Read the article Overview of Randomized Trials of Antithrombotic Therapy for COVID-19 Patients.
Find more COVID cardiology related news and video
COVID-19 Changes Properties Blood Cells